Novel Benzosuberone Derivatives:Synthesis, Characterization and Antibacterial Activity
J. Venkateswara Rao1 *, V. Krishna Reddy 1,2, Ram Bhavani2, Balram Bhavani2
1Department of Chemistry, Bapatla Engineering College, Bapatla-522101, Guntur (Dist), A.P., India
2Green Evolution Laboratories ,Wangapally Village, Nalgonda,500085, AP, India.
Corresponding Author Email: drjvraobec2013@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310451
Article Received on :
Article Accepted on :
Article Published : 08 Dec 2015
The synthesis of novel amide derivatives of benzosuberone 7a-j from commercially available benzosuberone was successfully achieved in six steps. Some of the important reactions that are involved in the synthesis are (i) insertion of methylester (ii) Suzki reaction and (iii) saponification followed by amide bond formation. The newly synthesis benzosuberone derivatives 7a-j were screened for antibacterial activity and the results indicated that in general, benzosuberone derivatives with R = piperazine ring showed good antibacterial activity.
KEYWORDS:Benzosuberone; Suzuki reaction; Saponification; Antibacterial activity
Download this article as:| Copy the following to cite this article: Rao J. V, Reddy V. K, Bhavani R, Bhavani B. Novel Benzosuberone Derivatives:Synthesis, Characterization and Antibacterial Activity. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Rao J. V, Reddy V. K, Bhavani R, Bhavani B. Novel Benzosuberone Derivatives:Synthesis, Characterization and Antibacterial Activity. Orient J Chem 2015;31(4). Rao J. V, Reddy V. K, Bhavani R, Bhavani B. Novel Benzosuberone Derivatives:Synthesis, Characterization and Antibacterial Activity. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=13138 |
Introduction
Benzosuberone derivatives possess potential bacteriostatic, anti-inflammatory, anti-pyretic, anti-ulcer, CNS-depressant, CNS stimulant and anti-convulsant activities. Some of the derivatives are also known for anti-tumor activity in murine P388 cell line tests [1]. Tricyclic antidepressants containing dibenzosuberone moieties mostly effect the autonomic and central nervous systems, and traditional anti-depressants, like amitriptyline [2], imipramine [3] and noxiptiline [4] which continue to be used as first-line drugs in treating depressive disorders.
Seven member ring fused to an aromatic ring is an important scaffold and has a become a integral part of certain natural products such as, in black tea having Theaflavin [5] as the main constituent and the plant Colchicum autumnal having Colchicine as the important ingredient with significant anti-cancer activity. Futhermore, Colchicine having the basic skeleton of benzosuberone was found to exhibit ant-tumour activity [6]. In recent years, a set of new benzosuberone derivatives embedded with coumarin and thiazolidindione frame work was found to exhibit in vitro anti-cancer activity and anti-proliferative property [7-9]. The present paper describes the synthesis, characterization and antibacterial activity of a set of new benzosuberone derivatives 7a-j, prepared from commercially available benzosuberone in five steps.
Results and Discussion
Chemistry
The synthesis of benzosuberone derivatives 7a-j is illustrated in scheme 1. Treatment of benzosuberone 1 with cyanoformate in presence of NaHMDS in THF at 0 oC for 2.5 h yielded in the corresponding methylester 2 in 85% yield. Reaction of compound 2 with N-Phenyl-bis(trifluoromethanesulfonamide)(PhNTf2) in presence of sodium bis(trimethylsilyl)amide in THF at -78 oC to room temperature gave the corresponding triflate derivative 3 in 70% yield. Suzuki reaction of triflate 3 with 3-benzoloxy benzene boric acid in presence of Pd2(dba)3, Na2CO3:water, in diethoxymethane, at 90oC for 2 h yielded benzoloxy methyl ester derivative 4 in 86% yield. Saponification of methyl ester 4 in presence of NaOH:water, in dioxane at 110 oC for 20 h resulted in the carboxylic acid 5 in 92% yield. Coupling of carboxylic acid 5 with secondary amines 6a-j in presence of EDC, HOBT, triethyl amine in dichloromethane at room temperature for 16 h resulted in the corresponding amide derivatives 7a-j. The structures of these compounds were established on the basis of 1H NMR, mass and IR data. The mass spectral data (M+1) of the compounds are in agreement with desired molecular formulae. As a example, the 1H NMR of compound 7b i.e (E)-5-(3-(benzyloxy)phenyl)-8,9-dihydro-N-methyl-7H-benzo[7]annulene-6-carboxamide is described here: The aromatic ring protons (13 H) appeared in the region 7.40 ppm – 6.78 ppm, while the characteristic benzoloxy methylene –CH2 signal appeared at 5.0 ppm as singlet and the –NH proton signal resonated at 5.18 ppm as a broad singlet. The seven membered methylene protons resonated at 2.56ppm, 2.30 ppm and 2.19 ppm. The mass spectrum of compound 7b showed molecular ion peak at m/z 384.3 M+1 peak.
Antibacterial activity
The results of the antibacterial activity of benzosuberone amides 7a-j is tabulated in table 1. Compounds 7g, 7h, 7i and 7j showed good activity, compounds 7a, 7b, 7e and 7f showed moderate activity and the remaining compounds 7c and 7d displayed weak activity, against the bacterial strains mentioned in the table 1.The zone of inhibitions of compounds 7a-j varied between 17-23 mm and was evaluated with respect to the drug Norfloxacin (zone of inhibitions 19- 25 mm). Based on the above results, it may be generalized that benzosuberone derivatives with R = piperazine ring exceptional being morpholine ring showed good antibacterial activity, while the remaining substituent’s in the series (R = -NH2, -NHMe, Pyrrolidine, Piperidine, -NMe2 and –N(CH2CH3)2 exhibited moderate to weak antibacterial activity. Based on the above generalization, it may be predicted that a further structural activity relationship may lead to a good antibacterial drug candidate.
Table 1: Antibacterial Screening Results of Compounds 7a-j
|
Compound No |
Gram negative bacteria |
Gram positive bacteria |
||
|
Escherichia coli |
Pseudomonas aeruginosa |
Staphylococcus aureus |
Streptococcus pyogenes |
|
|
Measurement of inhibition in mm |
||||
|
7a |
16 |
14 |
15 |
14 |
|
7b |
17 |
15 |
14 |
14 |
|
7c |
9 |
7 |
10 |
11 |
|
7d |
7 |
8 |
10 |
9 |
|
7e |
15 |
13 |
16 |
15 |
|
7f |
17 |
14 |
15 |
15 |
|
7g |
20 |
16 |
18 |
17 |
|
7h |
23 |
18 |
20 |
18 |
|
7i |
21 |
17 |
20 |
18 |
|
7j |
20 |
17 |
19 |
17 |
|
Norfloxacin a |
25 |
19 |
22 |
19 |
a.Concentration: 25 μg/mL of DMSO; b. No activity
Experimental
Standard operating procedures was implemented for the purification of solvents before being utilized for the reactions and work up’s. Merck silica gel 60 (230-400 mesh) and Merck pre-coated plates (silica gel 60 F254) was used for the routine column chromatography and visualization of spots (under UV lamp). Mel-temp apparatus
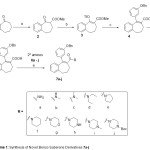 |
Scheme 1: Synthesis of Novel Benzo Suberone Derivatives 7a-j Reaction conditions: a) Cyanoformate, 1M NaHMDS, THF, 0 oC, 2.5 h; b) NaN3(TMS)2, PhNTf2, -78 oC – r.t, 16 h; c) 3-benzoloxy benzene boric acid, Pd2dba3, Na2CO3:water, diethoxymethane, 90oC, 2 h; d) NaOH:water, Dioxane, 110 oC, 20 h; e) 2oamines 6a-j, EDC, HOBT, TEA, DCM, r.t., 16 h; Click here to View scheme |
was utilized for the determination of melting point (m.p). Agilent ion trap MS was utilized for recording the mass spectra. Perkin Elmer FT-IR spectrometer was used for recording the IR data. Varian NMR-300 MHz instrument was used to record 1H NMR spectra. Chemical shifts values are measured in terms of δ ppm (parts per million) with reference to tetramethylsilane (TMS) as internal standard. The following notations viz., singlet-s, doublet-d, double doublet-dd, and multiplet-m was used for the signals that has appeared in the proton NMR spectrum and the coupling constant value was measured in terms of Hz.
methyl 6,7,8,9-tetrahydro-5-oxo-5H-benzo[7]annulene-6-carboxylate 2
A pre-mixed solution of sodium bis-(trimethylsilyl) amide (11.43 g, 62.45 mL, 62.45 mmol) in THF was added for 1h, to benzosuberone 1 (5 g, 31.25 mmol) dissolved in dry THF (50 mL), maintaining the temperature of the reaction at 0oC. Methyl cyanoformate (3.45 g, 40.58 mmol) was slowly added to the above contents and stirred for 1.5 h at the same same temperature. After completion of the reaction, as monitored by T.L.C, the reaction contents were diluted with saturated NH4Cl solution followed by EtOAc. The separated organic layer was worked up in a regular procedure that is extracting with ethylacetate and washing with water followed by bine solution and drying on anhydrous sodium sulphate. Concentration of the ethylacetate layer under reduced pressure resulted in the crude compound. Column chromatography of the crude compound was done (silica gel: 60-120 mesh; elluant: 1-2% EtOAc in pet ether) to afford compound 2. Pale yellow liquid; Yield: 5.8 g, 85%; 1H NMR (300 MHz, CDCl3): δ 12.60 (s, 1H), 7.66-7.58 (m, 1H), 7.36-7.28 (m, 2H), 7.23-7.18 (m, 1H), 3.82 (s, 3H), 2.95 (brs, 1H), 2.63 (t, J = 6.9 Hz, 2H), 2.10 (t, J = 7.2 Hz, 4H); ESI-MS: m/z, 219.3 (M+1).
(Z)-8-(methoxycarbonyl)-6,7-dihydro-5H-benzo[7]annulen-9-yl trifluoromethanesulfonate 3
Sodium bis(trimethylsilyl)amide (10.57 g, 57.8 mmol) was dissolved in THF and added to compound 2 (6.3 g, 28.89 mmol) that was previously dissolved in dry THF which was cooled at -78°C. The reaction contents was stirred for 1 h and then N-Phenyl-bis(trifluoromethanesulfonamide)(PhNTf2) (15.475 g, 43.34 mmol) was added to the above reaction mixture at -78°C and was allowed to stir for 2 h at -65 to -55 °C. After reaching the room temperature the reaction mixture was stirred for additional 16 h. After completion of the reaction, as monitored by T.L.C, the reaction contents were diluted with saturated NH4Cl solution followed by EtOAc. The separated organic layer was worked up in a regular procedure that is extracting with ethylacetate and washing with water followed by bine solution and drying on anhydrous sodium sulphate. Concentration of the ethylacetate layer under reduced pressure resulted in the crude compound. Column chromatography of the crude compound was done (silica gel: 60-120 mesh; elluant: 1-2% EtOAc in pet ether) to afford compound 3. Light yellow liquid; Yield: 7.1 g, 70%; 1H NMR (300 MHz, CDCl3): δ 7.48-7.40 (m, 4H), 3.90 (s, 3H), 2.72 (t, J = 7.2 Hz, 2H), 2.32-2.22 (m, 4H); ESI-MS: m/z, 291.5 (M+1).
(E)-methyl 5-(3-(benzyloxy)phenyl)-8,9-dihydro-7H-benzo[7]annulene-6-carboxylate 4
Compound 3 (7.1 g, 20.28 mmol) was dissolved in diethoxymethane (120 mL) and then the following reagents / catalyst and base was added sequentially 3-benzyloxy benzene boronic acid (6 g, 26.37 mmol), Na2CO3 (6.45 g, 60.85 mmol) followed by Pd2(dba)3 (0.0928 g, 0.10 mmol) and heated to reflux for 2 h. The reaction contents was diluted with water and extracted with EtOAc. After regular work up and column purification (silica gel:(60-120 mesh, elluent: 5% EtOAc in pet ether) compound 4 was isolated. Pale yellow Liquid; Yield: 6.7 g, 86%; IR (KBr): υmax 3061, 3027, 2939, 2859, 1709, 1580, 1531, 1485, 1434, 1381, 1348, 1317, 1277, 1236, 1194, 1114, 1091, 1029, 964, 909 cm-1; 1H NMR (300 MHz, CDCl3): δ 7.40-7.10 (ser.m, 9H), 6.93-6.72 (ser.m, 4H), 5.0 (s, 2H), 2.75 (t, J = 7.2 Hz, 2H), 2.38-2.20 (m, 4H); ESI-MS: m/z, 385.2 (M+1).
(E)-5-(3-(benzyloxy)phenyl)-8,9-dihydro-7H-benzo[7]annulene-6-carboxylic acid 5
Compound 4, (6.7 g, 17.44 mmol) was dissolved in 1,4-dioxane:H2O (130 mL, 3:1) and then NaOH (10.46 g, 261.71 mmol) was added and refluxed for 24 h. After completion of the reaction, the reaction contents was evaporated under reduced pressure to one-fourth volume, diluted with water, acidified with conc. HCl to obtain off-white solid that was filtered and dried at the pump to obtain compound 5 . The isolated product was utilized as such in the next. Off-white powder; Yield: 6.0 g, 92.9%; M.p.: 138-143 °C; IR (KBr): υmax 3057, 3023, 2931, 2858, 1676, 1585, 1489, 1425, 1382, 1318, 1282, 1238, 1201, 1158, 1085, 1014, 917, 867 cm-1; 1H NMR (300 MHz, CDCl3): δ 12.30 (brs, 1H), 7.46-7.20 (m, 9H), 6.96 (dd, J = 1.5, 6.0 Hz, 1H), 6.74 (dd, J = 1.2, 6.6 Hz, 2H), 6.66 (dd, J = 6.0 Hz, 1H), 5.02 (s, 2H), 2.76 (t, J = 7.2 Hz, 2H), 2.18-2.02 (m, 4H); ESI-MS: m/z, 370.2 (M+1).
General experimental procedure for the synthesis of compounds 7a-j
EDC.HCl (0.15g, 0.81 mmol), HOBt (0.11g, 0.81 mmol), triethylamine (0.151 mL, 1.077 mmol) and secondary amines 6a-j (0.0730 g, 1.0 mmol) was added consecutively to a stirred suspension of 5 (0.2 g, 0.540 mmol) dissolved in DCM (5.0 mL) and the reaction contents was stirred for 24 h at room temperature. After standard work up procedures, extraction with ethylacetate, washings with water, brine solution and drying on sodium sulphate and finally evaporation of the organic solvent and column chromatography purification (silica gel: 60-120 mesh), elluent: 0.5% of MeOH in CHCl3 resulted in the amide derivatives compound 6a-j. Yields of the compounds differed from 75-85%.
(E)-5-(3-(benzyloxy)phenyl)-8,9-dihydro-7H-benzo[7]annulene-6-carboxamide 7a
Off-white solid; Yield: 75%; M.p: 127-128 °C; IR (KBr): υmax 3290, 3046, 3027, 2932, 2866, 2788, 1639, 1536, 1474, 1442, 1384, 1322, 1282, 1228, 1158, 1094, 1038, 988, 942 cm-1; 1H NMR (300 MHz, CDCl3): δ 7.38-7.30 (m, 5H), 7.36-7.22 (m, 3H), 7.12 (td, J = 1.2, 6.6 Hz, 1H), 6.90 (dd, J = 1.5, 6.6 Hz, 1H), 6.80-6.78 (m, 3H), 5.04 (s, 2H), 3.78 (brs, 2H), 2.68 (t, J = 5.2 Hz, 2H), 2.56(t, J = 3.6 Hz, 3H), 2.28 (t, J = 6.0 Hz, 2H), 2.20 (t, J = 6.0 Hz, 2H); ESI-MS: m/z, 370.0 (M+1).
(E)-5-(3-(benzyloxy)phenyl)-8,9-dihydro-N-methyl-7H-benzo[7]annulene-6-carboxamide 7b
Off-white solid; Yield: 76%; M.p: 177-183 °C; IR (KBr): υmax 3295, 3049, 3022, 2929, 2859, 2793, 1631, 1532, 1476, 1448, 1391, 1328, 1285, 1225, 1152, 1090, 1034, 992, 950 cm-1; 1H NMR (300 MHz, CDCl3): δ 7.40-7.30 (m, 5H), 7.38-7.22 (m, 3H), 7.14 (td, J = 1.2, 6.6 Hz, 1H), 6.92 (dd, J = 1.5, 6.6 Hz, 1H), 6.84-6.78 (m, 3H), 5.18 (brs, 1H), 5.0 (s, 2H), 2.70 (t, J = 5.2 Hz, 2H), 2.56 (d, J = 3.6 Hz, 3H), 2.30 (t, J = 6.0 Hz, 2H), 2.19 (t, J = 6.0 Hz, 2H); ESI-MS: m/z, 384.3 (M+1).
(E)-5-(3-(benzyloxy)phenyl)-8,9-dihydro-N,N-dimethyl-7H-benzo[7]annulene-6-carboxamide 7c
White solid; Yield: 78%. M.p: 131-132 °C; IR (KBr): υmax 3292, 3042, 3025, 2936, 2867, 2798, 1633, 1528, 1472, 1444, 1388, 1320, 1282, 1219, 1150, 1102, 1028, 984, 944 cm-1; 1H NMR (300 MHz, CDCl3): δ 7.42-7.32 (m, 5H), 7.38-7.22 (m, 3H), 7.16 (td, J = 1.2, 6.6 Hz, 1H), 6.92 (dd, J = 1.5, 6.6 Hz, 1H), 6.86-6.78 (m, 3H), 5.02 (s, 2H), 3.10 (s, 6H), 2.72 (t, J = 5.2 Hz, 2H), 2.30 (t, J = 6.0 Hz, 2H), 2.20 (t, J = 6.0 Hz, 2H); ESI-MS: m/z, 398.3 (M+1).
(E)-5-(3-(benzyloxy)phenyl)-N,N-diethyl-8,9-dihydro-7H-benzo[7]annulene-6-carboxamide 7d
Oily liquid; Yield: 82%; 1H NMR (300 MHz, CDCl3): δ 7.41-7.29 (m, 5H), 7.44-7.24 (m, 3H), 7.16 (td, J = 1.2, 6.6 Hz, 1H), 6.90 (dd, J = 1.5, 6.6 Hz, 1H), 6.84-6.78 (m, 3H), 5.0 (s, 2H), 3.60 (q, J = 5.2, 4H), 2.70 (t, J = 5.2 Hz, 2H), 2.30 (t, J = 6.0 Hz, 2H), 2.19 (t, J = 6.0 Hz, 2H), 1.27 (t, J = 5.4 Hz, 6H); ESI-MS: m/z, 426.0 (M+1).
((E)-9-(3-(benzyloxy)phenyl)-6,7-dihydro-5H-benzo[7]annulen-8-yl)(pyrrolidin-1-yl)methanone 7e
Pale yellow viscous; Yield: 80%; 1H NMR (300 MHz, CDCl3): δ 7.44-7.32 (m, 5H), 7.408-7.24 (m, 3H), 7.18 (td, J = 1.2, 6.6 Hz, 1H), 6.88 (dd, J = 1.5, 6.6 Hz, 1H), 6.84-6.78 (m, 3H), 5.02 (s, 2H), 3.57 (brs, 4H), 2.72 (t, J = 5.2 Hz, 2H), 2.32 (t, J = 6.0 Hz, 2H), 2.24 (t, J = 6.0 Hz, 2H), 2.08 (brs, 4H); ESI-MS: m/z, 424.3 (M+1).
((E)-9-(3-(benzyloxy)phenyl)-6,7-dihydro-5H-benzo[7]annulen-8-yl)(piperidin-1-yl)methanone 7f
Yellow viscous; Yield: 85%; 1H NMR (300 MHz, CDCl3): δ 7.36-7.32 (m, 5H), 7.34-7.20 (m, 3H), 7.16 (td, J = 1.2, 6.6 Hz, 1H), 6.92 (dd, J = 1.5, 6.6 Hz, 1H), 6.86-6.80 (m, 3H), 5.0 (s, 2H), 3.87 (t, J = 3.6, 4H), 2.70 (t, J = 5.2 Hz, 2H), 2.30 (t, J = 6.0 Hz, 2H), 2.19 (t, J = 6.0 Hz, 2H), 1.72-1.59 (m, 6H); ESI-MS: m/z, 438.3 (M+1).
((E)-9-(3-(benzyloxy)phenyl)-6,7-dihydro-5H-benzo[7]annulen-8-yl)(morpholino)methanone 7g
Pale yellow viscous; Yield: 85%; 1H NMR (300 MHz, CDCl3): δ 7.40-7.30 (m, 5H), 7.38-7.22 (m, 3H), 7.14 (td, J = 1.2, 6.6 Hz, 1H), 6.92 (dd, J = 1.5, 6.6 Hz, 1H), 6.84-6.78 (m, 3H), 5.02 (s, 2H), 3.87 (t, J = 3.6, 4H), 3.64 (t, J = 3.9 Hz, 4H), 2.70 (t, J = 5.2 Hz, 2H), 2.30 (t, J = 6.0 Hz, 2H), 2.19 (t, J = 6.0 Hz, 2H), ESI-MS: m/z, 443.1 (M+1).
((E)-9-(3-(benzyloxy)phenyl)-6,7-dihydro-5H-benzo[7]annulen-8-yl)(piperazin-1-yl)methanone 7h
Pale yellow viscous liquid; Yield: 84%; 1H NMR (300 MHz, CDCl3): δ 7.40-7.30 (m, 5H), 7.38-7.22 (m, 3H), 7.14 (td, J = 1.2, 6.6 Hz, 1H), 6.92 (dd, J = 1.5, 6.6 Hz, 1H), 6.84-6.78 (m, 3H), 5.02 (s, 2H), 3.10 (t, J = 3.6, 4H), 2.82 (brs, 4H), 2.70 (t, J = 5.2 Hz, 2H), 2.30 (t, J = 6.0 Hz, 2H), 2.19 (t, J = 6.0 Hz, 2H), ESI-MS: m/z, 441.3 (M+1).
((E)-9-(3-(benzyloxy)phenyl)-6,7-dihydro-5H-benzo[7]annulen-8-yl)(4-ethylpiperazin-1-yl)methanone 7i
Yellow oily liquid; Yield: 78%; 1H NMR (300 MHz, CDCl3): δ 7.40-7.30 (m, 5H), 7.38-7.22 (m, 3H), 7.14 (td, J = 1.2, 6.6 Hz, 1H), 6.92 (dd, J = 1.5, 6.6 Hz, 1H), 6.84-6.78 (m, 3H), 5.0 (s, 2H), 3.68 (t, 3.6 Hz, 4H), 2.70 (t, J = 5.2 Hz, 2H), 2.59 (t, J = 3.6 Hz, 4H), 2.30 (t, J = 6.0 Hz, 2H), 2.40 (q, J = 5.4 Hz, 2H), 2.19 (t, J = 6.0 Hz, 2H), 1.13 (t, J = 5.1 Hz, 3H); ESI-MS: m/z, 467.0 (M+1).
((E)-9-(3-(benzyloxy)phenyl)-6,7-dihydro-5H-benzo[7]annulen-8-yl)(t-butyl-piperazin-1-yl)methanone 7j
Yellow syrupy liquid; Yield: 85%; 1H NMR (300 MHz, CDCl3): δ 7.40-7.30 (m, 5H), 7.38-7.22 (m, 3H), 7.14 (td, J = 1.2, 6.6 Hz, 1H), 6.92 (dd, J = 1.5, 6.6 Hz, 1H), 6.84-6.78 (m, 3H), 5.02 (s, 2H), 3.64 (brs, 4H), 3.60 (brs, 4H), 2.70 (t, J = 5.2 Hz, 2H), 2.30 (t, J = 6.0 Hz, 2H), 2.19 (t, J = 6.0 Hz, 2H), 1.48 (s, 9H); ESI-MS: m/z, 539.0 (M+1).
Anti-microbial activity assay
Benzosuberone derivatives 7a–j were tested against the bacterial strains viz., i) Escherichia coli (MTCC443), (ii) Pseudomonas aeruginosa (MTCC424), (Gram negative bacteria) and (iii) Staphylococcus aureus (MTCC96) iv) Streptococcus pyogenes (MTCC442), (Gram positive bacteria) using agar diffusion method according to the literature protocol [10-12]. Benzosuberone derivatives 7a–j were dissolved in dimethyl sulphoxide at 25 μg/mL concentration. Paper discs (6 mm, punched from Whatmann no 1 paper) were ultraviolet sterilized and used for the assays. Discs were soaked in different concentration of the test solution and placed on the inoculated agar media at regular intervals of 6-7 cm, taking care to ensure that excess solution was not left on the discs. The plates were incubated at 37oC in an inverted fashion. Activity was determined by zones showing complete inhibition (mm). Growth inhibition was calculated with reference to positive control. All the samples were taken in triplicate.
Conclusion
The newly synthesized benzosuberone derivatives 7a-j was determined by spectroscopic techniques like 1H NMR, mass and IR. From the results of the antibacterial screening data, it was evident that compounds 7g, 7h, 7i and 7j showed good activity and the remaining compounds showed moderate to weak activity.
Acknowledgements
One of the authors (KR) is thankful to Dr. B. Ram, the Director, Green Evolution Laboratories for their helpful suggestions and constant encouragement
References
- National Cancer Institute, Bethesda, MD 24014, 1971.
- Hoffsomer, R.D.; Taub, D.; Wendler, N.L. J. Org. Chem. 1962, 27, 4134.
- Schindler, W.; Hafliger, F.; Uber. Helv. Chim. Acta. 1954, 59, 472.
- Hoffmeister, V.F.; Wutke, W.; Kroneberg, G. Arzneim. Forsch. 1969, 19, 846.
- Yang, C. S.; Lambert, J. D.; Ju, J.; Lu,G.; Sang, S. Toxicol. Appl. Pharmacol. 2007, 2, 265.
- Chaplin, D. J.; Hills, S. A. Int. Radiat. Oncol. Biol. Phys. 2002, 54, 1491.
- Nagarapu, L.; Yadagiri, B.; Bantu, R.; Kumar, C. G.; Pombala, S.; Nanubolu, J. Eur.J. Med. Chem. 2014, 71, 91.
- Yadagiri, B.; Holagunda, U. D.; Bantu, R.; Nagarapu, L.; Kumar, C. G.; Pombala, S.; Sridhar, B. Eur. J. Med. Chem. 2014, 79, 260.
- Yadagiri, B.; Holagunda, U.D.; Bantu, R.; Nagarapu, L.; Guguloth, V.; Polepally, S.; Jain N. Bioorg. Med. Chem. Lett. 2014, 24, 5041–5044
- Bauer, A. N.; Kirby, W. N.; Sherries, J.C.; Truck, M. Am. J. Clin. Pathol, 1966, 45, 493.
- Pandey, K. S.; Khan, N. Arch Pharm Chem Life Sci. 2008, 341, 418.
- Atta-ur-Rahman, M.I.; Choudhary, W.J. Thomsen, Bioassay techniques For Drug Development (Harwood Academic Publishers, Amsterdam, The Netherlands), 2001, 22, 16.

This work is licensed under a Creative Commons Attribution 4.0 International License.









