Functionalization of Multi-Walled Carbon Nanotubes by 1-Amino-2-Naphthol-4-Sulfonic Acid and Study of Their Antimicrobial Activity Against The Gram-negative and Gram-positive Bacterias.
Sadaf Sami1 and Elaheh Rahimpour*2
1Department of Chemistry, Islamic Azad University, North Tehran Branch, Tehran (Iran)
2Department of Chemistry, Azarbaijan Shahid Madani University, Tabriz (Iran)
Corresponding Author Email: rahimpour@azaruniv.edu
DOI : http://dx.doi.org/10.13005/ojc/310442
Article Received on :
Article Accepted on :
Article Published : 06 Dec 2015
In this study, we investigated the chemical functionalization of carboxylated multiwalled carbon nanotubes (MWCNT–COOH) by 1-Amino-2-naphthol-4-sulfonic acid (ANSA). The functionalized MWCNTs exhibited good aqueous solubility due to the presence of the –OH and –SO3H groups of ANSA. The antimicrobial activity of the MWCNT–COOH as well as its functionalized multiwall nanotube MWCNT-CO-2-(1-amino-2-naphthol-4-sulfonic acid) was tested against some gram-negative and gram-positive bacterias. In vitro studies, MWCNT−ANSA represented a significant enhancement in the antimicrobial capability of MWCNTs against gram-negative E. coli and S. typhimurium bacterias. Therefore, these functionalized carboxylated multi wall nanotubes could potentially be considered as antimicrobial candidates.
KEYWORDS:Carboxylated multiwall nanotubes; Functionalization; 1-Amino-2-naphthol-4-sulfonic acid; Antimicrobial activity
Download this article as:| Copy the following to cite this article: Sami S, Rahimpour E. Functionalization of Multi-Walled Carbon Nanotubes by 1-Amino-2-Naphthol-4-Sulfonic Acid and Study of Their Antimicrobial Activity Against The Gram-negative and Gram-positive Bacterias. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Sami S, Rahimpour E. Functionalization of Multi-Walled Carbon Nanotubes by 1-Amino-2-Naphthol-4-Sulfonic Acid and Study of Their Antimicrobial Activity Against The Gram-negative and Gram-positive Bacterias. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=13062 |
Introduction
With changing food production and eating habits, new pathogens and newly recognized vehicles of infection have emerged. Most of mortality throughout the world occurs due to infectious diseases 1. Concerns about contagious spread of infection are growing and the performed studies in this regard are abundant 2,3.
Carbon nanotubes (CNTs) have been recently demonstrated to possess antimicrobial properties, and their relevant activities were attributed to the behavior of ‘nanodart’ with the proposed physical damage mechanism 4. But the antimicrobial activity of CNTs is limited due to chemical–physical properties such as the size of two terminals. To improve the antimicrobial activity of CNTs, functionalization with various groups such as metallic nanoparticles 5 or lysozymes 6 has been reported. However, the stability of silver ions or lysozymes on the CNTs surface is low. It can be ascribed to physical adsorption these functional groups that resulted in antimicrobial efficacy of CNTs loss. Chemical functionalization is a common technique to improve the antimicrobial activity of CNTs 7,8. Covalent functionalization with cationic charge chemical groups are often employed, which exert activity through attack on the bacterial membrane. Furthermore, chemical functionalization enhance dispersion stability and biocompatibility of CNTs 9. The covalent modifications of nanotubes have been described in some review papers 10,11.
1-Amino-2-naphthol-4-sulfonic acid (ANSA) is a tri-functional monomer, i.e. bearing three functional groups (–NH2, –OH and –SO3H), and contains two fused benzene rings 12 Free –NH2 group can combine with functional groups of MWCNTs and the existence of the –OH and –SO3H groups in ANSA plays an important role in improving the solubility of the MWCNTs in common polar solvents.
In this study, we attached the 1-amino-2-naphthol-4-sulfonic acid to the surface of carboxylated multiwall nanotubes. The resulted product MWCNT-ANSA were characterized by Fourier transform infrared spectra (FT-IR), Raman and transmission electron microscopy (TEM). MWCNT-ANSA display improved antimicrobial activities against Gram-negative bacteria of Escherichia coli and Salmonella typhimurium bacteria.
Experimental
Material and Methods
Multi-walled carbon nanotubes MWCNT– COOH (95 % purity, 5–10 nm) were purchased fromNetrino Co. Ltd. All other chemicals were purchased from Merck and used without further purification.
A Cintra-20 double-beam UV–vis spectrophotometer (GBC Scientific Equipment, Victoria, Australia) and Nexus 870 Fourier transform infrared spectrometer (Thermo Nicolet, Madison, WI) were employed for the UV‒vis and FT-IR spectral measurements, respectively. The TEM measurements were performed by using a transmission electron microscope model EM208 (Philips, the Netherlands). FT‒Raman spectra were recorded on a 960 ES spectrometer (Thermo Nicolet, Madison, WI). All of the measurements were carried out at room temperature.
Ultrasonic treatment was provided by a model UP 200 S ultrasonic bath (Hielscher, Germany). An electronic analytical balance model PB 303 (Mettler Toledo, Switzerland) was used for weighting the solid materials.
Preparation of MWCNT–COCl
The nanotubes were all treated first to attach chlorine groups to their surface. The chlorination procedure was the following. A 200-mg of nanotubes was added to 20 mL of thionyl chloride in acidic media. The mixture was sonicated in an ultrasonic bath for 30 min at ambient temperature. Then the mixture was refluxed for 24 h at 80 ◦C. After cooling to room temperature, the mixture was filtered through a PTFE filter (10-μm pore size), and washed thoroughly with tetrahydrofuran. Subsequently, the product was dried in an oven at 70 ◦C for 4 h.
Preparation of 1-amino-2-naphthol-4-sulfonic acid‒functionalized MWCNTs
The chlorineted nanotubes was dispersed by sonication in 30 mL of Dimethylformamide (DMF) for 30 min. 400 mg of the 1-amino-2-naphthol-4-sulfonic acid was then added. The solution was refluxed for 48 h at 80 ◦C. The resulted suspension was filtered by PTFE filter (0.2-μm pore size) and to remove unreacted reagent, the filter cake was thoroughly washed with ethanol by three times, after that with 100 mL tetrahydrofuran (THF). The black solid, with sulfonic acid groups attached to the MWCNT (Scheme 1), was then dried at room temperature under vacuum.
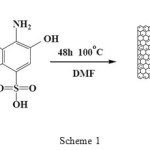 |
Scheme 1: Preparation of ANSA functionalized MWCNTs. |
Antimicrobial Susceptibility Testing
Susceptibility tests were performed by the disc diffusion method of Bauer et al. 13 with Mueller-Hinton agar (Difco). A bacteria culture (which has been adjusted to 0.5 McFarland standard 14), was used to lawn Muller Hinton agar plates evenly using a sterile swab. Plates supplemented with 2% glucose and Methylene Blue (0.5 mg/L). The discs which had been impregnated with ANSA, MWCNTs‒COOH and ANSA functionalized‒ MWCNTs in the concentration range of 1000−3000 mg L−1 were placed on these plates. Each plate had two treated discs placed about equidistance from plate wall. Zones of inhibition, where growth decreased abruptly, were measured after 24 h of incubation at 35 ◦C. The tests were repeated three times to ensure reliability. Staphylococcus aureus (S. aureus), Bacillus cereus (B. cereus), Escherichia coli (E. coli), Salmonella typhimurium (S. typhimurium) were used for antimicrobial assays.
Results and Discussion
Characterization of 1-amino-2-naphthol-4-sulfonic acid functionalized‒ MWCNTs
A set of characterization was performed in order to get better insight into the structural properties and morphology of the 1-amino-2-naphthol-4-sulfonic acid functionalized‒ MWCNTs.
Infrared Spectroscopy
The infrared absorption spectroscopy was used to identify the nature and symmetry of surface functionalized groups. In the FT-IR spectrum (Fig. 1) the two bands indicative of C=O and C–O stretching vibrations in 1704 and 1204 cm-1 give valuable information about the behavior of the attached functional groups to the MWCNTs. The IR spectrum of the ANSA-functionalized MWCNT samples, MWCNT-CO-HNC10H5(OH)SO3H (Fig. 1a), shows the disappearance of the band at 1704 cm-1 and corresponding appearance of a band with lower frequency (1645 cm-1) assigned to the amide carbonyl (C=O) stretch 15. In addition, the presence of new bands at 3449 and 1221 cm-1, corresponding to N-H and C-N bond stretching, respectively, further confirms the presence of the amide functional group. The bands at 1357 cm−1 and 1166 cm−1 can be ascribed to asymmetric and symmetric of –SO2– bending vibrations 16. Also, the FT-IR spectrum shows characteristic bands at 655 and 754 cm−1 which can be assigned to the aromatic C–H out-of-plane bending vibrations and the weak band at around 889 cm−1 can be assigned to C–H out-of-plane bending vibration for one isolated hydrogen in the pentasubstituted benzene ring 12. These results indicate that the COOH‒MWCNTs have successfully modified by 1-amino-2-naphthol-4-sulfonic acid.
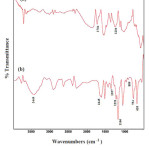 |
Figure 1: FT-IR spectra of MWNT–COOH (a) and ANSA functionalized MWCNTs (b). Click here to View figure |
Raman Spectroscopy
Raman spectroscopy is a powerful technique used to characterize structural changes of carbon nanotubes and provided information about MWCNT–COOH before and after functionalization. As shown in Fig. 2, the D and G bands of the MWCNT at 1279 and 1594 cm-1, attributed to defects, disorder-induced peaks, and tangential-mode peaks, can be clearly observed for both MWCNT–COOH and functionalized MWCNTs. More importantly, D- to G-band intensity ratio (ID/IG) for MWCNT‒COOH is around 0.736. After functionalization, some sp2 hybridized carbons in CNTs converted to be in sp3 hybridization 17,18. Therefore, the D/G ratio of modified MWCNTs increased to 1.402. This change in D/G ratio is used as an evidence of the disruption of the aromatic system of π electrons by the attached molecules and indicated the success of 1-amino-2-naphthol-4-sulfonic acid bonding to MWCNT‒COOH.
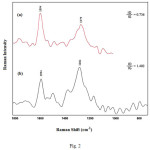 |
Figure 2: Raman spectra of MWCNT–COOH (a) and ANSA functionalized MWCNTs (b). Click here to View figure |
TEM Characterization
More direct evidence for the functionalization of MWCNTs is manifested by the TEM images. Fig. 3 clearly indicates that the MWCNT–COOH has a relatively smooth and clean surface. The changes in morphology of product are remarkable. A uniform tubular layer due to a covalently bonded aromatic group on the surface of the MWCNT (the rough part) is observable.
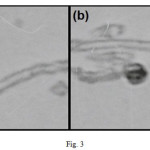 |
Figure 3: TEM images for MWCNT–COOH (a) and ANSA functionalized MWCNTs (b). Click here to View figure |
Solubility of Functionalized MWCNTs
Pristine MWCNTs and MWCNT‒COOH were either insoluble or little soluble in polar solvents and were difficult to evenly disperse in an aqueous matrix. Controlling the degree of dispersion of nanotubes is difficult because of the strong intra-molecular forces that exist between carbon nanotubes which are responsible for the formation of nanotube bundles 19. They were often sonicated to force their dispersion in the medium before any biology-related application. Herein, the existence of extremely large hydrophilic groups containing sulfonic acid in modified MWCNTs gives good dispersion in water to obtain a homogenous distribution of modified MWCNTs in solvent. The dispersion is stable for almost a month.
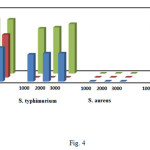 |
Figure 4: The diameters of the inhibitory zones for discs treated by MWCNT–COOH, ANSA and ANSA functionalized MWCNTs against some Gram-negative and Gram-positive bacteria. Click here to View figure |
Antimicrobial Assay
The in vitro antimicrobial activity of 1-amino-2-naphthol-4-sulfonic acid, MWCNT–COOH as well as corresponding functionalized MWCNTs against E. coli, S. typhimurium (Gram-negative bacteria) and S. aureus, B. cereus (Gram-positive bacteria) were determined by the disc diffusion method. As shown in Fig. 4, the diameter of the inhibitory zones for ANSA functionalized MWCNTs against Gram-negative bacteria E. coli enhances by 1.62 and 1.28 times compared to MWCNT–COOH and 1-amino-2-naphthol-4-sulfonic acid, respectively. Also, increases of 1.68 times against Gram-negative bacteria S. typhimurium compared to MWCNT–COOH were observed. It must be mentioned that 1-amino-2-naphthol-4-sulfonic acid have not any antimicrobial activity against S. typhimurium. However, no zones of inhibition was observed to gram-positive bacteria S. aureus and B. cereus.
The above mentioned data suggest that modified MWCNT is more effective against Gram-negative bacteria. It can be attributable to the different structures of cell walls of these bacteria 20. Since the antimicrobial ability of MWCNT depends on direct interaction with the bacterial membrane, the sulfono termination allows further chemistry of the functionalized MWCNTs 21,22 and makes possible electrostatic adsorption on bacteria membrane or permeability across the membrane and decrease metabolic activity and finally kill the bacteria.
Conclusions
In summary, a chemical reaction occurred between 1-amino-2-naphthol-4-sulfonic acid molecules and MWCNT, resulting in the attachment of ANSA to the carboxylic groups on the nanotube surface via amide formation. Covalent functionalization procedure could significantly increase aqueous solubility, which is a critical criterion for various applications. Antimicrobial activity of MWCNT-ANSA was studied on two Gram-positive and Gram-negative bacteria by disc diffusion method. The results shown strongest antimicrobial activity of MWCNT-ANSA compared to ANSA and the pristine MWCNT. Also, improved antimicrobial activity just for the gram-negative bacteria confirmed that mechanism of the antimicrobial activity of functionalized MWCNT is electrostatic adsorption of MWCNT-ANSA on the bacteria membrane and decreasing metabolic activity and finally killing the bacteria.
Acknowledgements
The financial support from the Research Council of Islamic Azad University is grate-fully acknowledged.
References
- Cohen, M.L. Nature 2000, 406, 762-767
- Aslan, S.; Loebick, C.Z.; Kang, S.; Elimelech, M.; Pfefferle L.D.; Tassel, P.R.V. Nanoscale 2010, 2, 1789-1794
- Amiri, A.; Zardini, H.Z.; Shanbedi, M.; Maghrebi, M.; Baniadam M.; Tolueinia, B. Mater. Lett. 2012, 72, 153-156
- Zhou, J.; Qi, X. Lett. Appl. Microbiol., 2010, 52, 76-83
- Mohan, R.; Shanmugharaj A.M.; Hun, R.S.; J. Biomed. Mater. Res. 2011, 96B, 119-126
- Nepal, D.; Balasubramanian, S.; Simonian A.L.; Davis, V.A. Nano Lett. 2008, 8, 1896-1901
- Entezari, M.; Safari, M., Hekmati, M., Hekmat S., Azin, A. Med. Chem. Res. 2014, 23, 487-495
- Azizian, J.; Tahermansouri, H.; Biazar, E.; Heidari S.; Khoei, D.C. Int. J. Nanomedicine 2010, 5, 907-914
- Georgakilas, V.; Kordatos, K.; Prato, M.; Guldi, D.M.; Holzinger M.; Hirsch, A. J. Am. Chem. Soc. 2002, 124, 760-761
- Banerjee, S.; Hemraj-Benny T.; Wong, S. S. Adv. Mater. 2005, 17, 17-29
- Dyke C.A., Tour, J.M. J. Phys. Chem. A 2004, 108, 11151-11159
- Bhandari, H.; Bansal, V.; Choudhary V.; Dhawan, S.K. Polym. Int. 2009, 58, 489-502
- Bauer, A.W.; Kirby, W.M.M.; Sherris J.C.; Turck, M. Amer. J. Clin. Pathol. 1966, 45, 493-496
- Zaidan, M.R.S.; Noor-Rain, A.; Badrul, A.R.; Adlin, A.; Norazah A.; Zakiah, I. Trop. Biomed. 2005, 22, 165-170
- Ramanathan, T.; Fisher, F.T.; Ruoff R.S.; Brinson, L.C. Chem. Mater. 2005, 17, 1290-1295
- Liu, Y.L.; Chen W.H.; Chang, Y.H. Carbohydr. Polym. 2009, 76, 232-238
- Jorio, A.; Saito, R.; Dresselhaus G.; Dresselhaus, M.S. Phil. Trans. R. Soc. Lond. A 2004, 362, 2311-2336
- Dresselhaus, M.S.; Dresselhaus, G.; Saito R.; Jorio, A. Phys. Rep. Rev. Sect. Phys. Lett. 2005, 409, 47-99
- Szymczyk, A.; Roslaniec, Z.; Zenker, M.; García-Gutiérrez, M.C.; Hernández, J.J.; Rueda, D.R.; Nogales A.; Ezquerra, T.A. Express Polym. Lett. 2011, 5, 977995
- Huang, K.C.; Mukhopadhyay, R.; Wen, B.; Gitai Z.; Wingreen, N.S. Proc. Natl. Acad. Sci. U S A 2008, 105, 19282-19287
- Sendai, M.; Hashiguchi, S.; Tomimoto, M.; Kishimoto, S.; Matsuo, T.; Kondo M.; Ochiai, M. J. Antibiot. 1985, 38, 346-371
- Sendai, M.; Hashiguchi, S.; Tomimoto, M.; Kishimoto, S.; Matsuo T.; Ochiai, M. Chem. Pharm. Bull. 1985, 33, 3798-3810

This work is licensed under a Creative Commons Attribution 4.0 International License.









