Chemical composition of the essential oils for AnthemisMelampodina from North Saudi Arabia
Jehan Al-humaidi
Chemistry Department, College of Science, Princess Nora Bint Abdul Rhman University,
DOI : http://dx.doi.org/10.13005/ojc/310453
Article Received on :
Article Accepted on :
Article Published : 04 Nov 2015
The chemical composition of essential oil of Anthemsmelampodinais determined by GC/MS. The oil was obtained by hydro-distillation and SPME extraction methodsIn the SPME method, a total of 41 constituents were identified monoterpene hydrocarbons (88.89%) were the main class of compounds detected in the SPME method with b-pinene(35.29%), trans-ocimene(23.96%) andterpinolene(15.78%) being detected as the main constituents. On the other hand, hydro-distilled oil was rich in oxygenated sesquiterpene(31.22%).
KEYWORDS:Anthems melampodina; Hydro-distillation; SPME; GC/MSmonoterpene hydrocarbons; oxygenated sesquiterpenes
Download this article as:| Copy the following to cite this article: Al-humaidi J. Chemical composition of the essential oils for AnthemisMelampodina from North Saudi Arabia. Orient J Chem 2015;31(4). |
| Copy the following to cite this URL: Al-humaidi J. Chemical composition of the essential oils for AnthemisMelampodina from North Saudi Arabia. Orient J Chem 2015;31(4). Available from: http://www.orientjchem.org/?p=12185 |
Introduction
Anthemis(belonging to the Astraceae family)is a genus of 210 species of flowering plants. Anthemis plants are known to growwidely in Europe, south western Asia, northern and north eastern Africa and southern Arabia.The arabic names for this plant include Qahwiyan,Rebyan and Arbiyar[1]. In Saudi Arabia, the genus Anthemis is represented by 17 species distributed in the central, eastern and northern regions[2]. examples include A. pseudocotula,A. bornmuelleri,A. cotula.A. odontostephenaand A. melampodina[3]. In folk medicine Anthemis species are frequently used for the treatment of various ailments such as digestive problems, insomnia and toothache.Moreover,several studies also showed that different Anthemis species exhibited antiflammatory, antioxidant, antibacterial, antiproliferative and antispasmodic properties[4-8]. Most Anthemisspecies are economically important, due to their use in pharmaceutics, cosmetics and food flavoring[9-13]. Furthermore, chemical investigations on Anthemisspecies revealed that the genus is dominated by sesquiterpene lactones, flavonoids and polyacetylenes[14-20].Anethimsmelampodina is anannual, greyish- tomentose, herb with ascending branches from near the base. The flowers are white-yellow in color.This plant is known to grow widely in northern region of Saudi Arabia including Hail[21].
In a continuation of an extensive work aimed at the investigation of the volatile constituents of aromatic plants from Saudi Arabia, the current investigation was designed to investigate the chemical composition of the essential oil A. melampodina obtained by two different extraction methods, the Solid Phase Micro-Extraction and hydro-distillation methods and compare the current findings with those obtained from other locations of the world.
Experimental
Plant Material
Aerial parts of A. melampodina were collected during the full flowering stage March 2012) from Hart Alrha – south Tabuk. The identity of the plant species was confirmed by Dr Jacob Thomas from the Herbarium Division, College of Science, King Saud University, Riyadh, KSA. A voucher specimen Anthps-PNU-013was kept in the Chemistry Department, College of Science, Princess NouraBint Abdel Rhman University, Riyadh, Saudi. The plant material was dried at room temperature until constant weight was obtained.
Hydro-distillation of plant material
Air dried flowering parts (150 g) were coarsely powdered and then hydro-distilled using a Clevenger apparatus for 3 h. The extraction was repeated twice and the obtained oils were pooled separately, dried over anhydrous sodium sulfate (Na2SO4) and stored at 4ºC in amber glass vials until analysis.
Solid Phase Micro Extraction of the volatile oils (SPME)
The Solid Phase Micro Extraction experiments were performed using SPME fiber assembly (Polydimethylsilocane/Divenylbenzene, PDMS/DVB; df 65 mm partially crossed-linked phase, fiber length 1 cm) and assemblies for manual sampling (Supelco, Bellefonte, PA, USA). Before measurements, the fiber was conditioned according to the producer’s recommendations. About 0.1 mg of freshly powdered flowers was introduced into 4.0 mL amber glass vials, tightly capped with PTFE-coated septa, and SPME extraction was performed for 2.0 min at room temperature. Desorption of the analytes was carried out at 240ºC for 60 s. Each sample was repeated twice.
GC-MS and GC-FID analysis
About 1 μl aliquot of each oil sample, diluted to 5 μl in GC grade n-hexane, was subjected to GC/MS analysis. The GC/MS analysis was performed using Varian Chrompack CP-3800 GC/MS/MS-200 (Saturn, Netherlands) equipped with DP-5 (5% diphenyl, 95% dimethyl polysiloxane) GC capillary column (30 m × 0.25 mm i.d., 0.25 μm film thicknesses), with helium as a carrier gas (flow rate 0.9 mL/min). The actual temperature in MS source was 180ºC and the ionization voltage was 70 eV. The column temperature was kept at 60ºC for 1 min (isothermal), and programmed to 246ºC at a rate of 3ºC/min, and kept constant at 246ºC for 3 minutes (isothermal). A hydrocarbon mixture of n-alkanes (C8-C20) was analyzed separately by GC/MS under the same chromatographic conditions using the same DP-5 column.
For the quantitative analysis (% area), a Hewlett-Packard HP-8590 gas chromatograph equipped with a split-splitless injector (split ratio 1:50) and an FID detector was used. The column was an optima-5 (5% diphenyl, 95% dimethyl polysiloxan) fused silica capillary column (30 m × 0.25 mm, 0.25 film thickness). The temperature of the oven was increased at a rate of 10ºC/min from 60ºC to 250ºC and then held constant at 250ºC for 5 min. The temperatures of the injector and detector were maintained at 250ºC and 300ºC, respectively. The relative peak areas of the oil components were measured and then used to calculate the concentration of the detected compounds. Each sample was analyzed twice.
Identification of the components
The components of the essential oils obtained from the SPME and hydro-distilled flowering parts of the plant were identified using the built in libraries (Nist Co and Wiley Co, USA) and by comparing their calculated retention indices relative to (C8-C20) n-alkanes literature values measured with columns of identical polarity (Adams, 2001), or with authentic samples. The compounds, α– and β-pinenes, p-cymene, limonene, linalool (Fluka, Buchs, Switzerland) and sabinene hydrate (Sigma-Aldrich, Buchs, Switzerland) were used as reference substances in GC/MS analysis. GC-grade hexane and analytical reagent grade anhydrous Na2SO4 were purchased from Scharlau (Barcelona, Spain) and UCB (Bruxelles, Belgium).
Results
The GC/MS analysis of the volatile potentials and hydrodistilled oil obtained from flowering parts of A. melampodinaled to the characterization of total of 76 different components(Table 1).In the SPME method, 47 components amounting to 98.25 % of the total oil content were identified. Monoterpene hydrocarbons(88.89%)were the main contributors to the SPME volatiles and were dominated by b-pinene (35.29%), trans-ocinene (23.29%), terpinolene (15.7%), g-terpinene (6.84%) and cis-ocimene (5.23%). Oxygenated monoterpenes accounted for 4.95% of the total oil content with tetrahydrolavanduledetected as a major components (0.94%).Sesquiterpene hydrocarbons were detected at much lower concentrations (2.51%) and the main contributors included b-sesquiphellandrene (1.23%) and guaiene (0.58%). In addition, oxygenated sesquiterpenes and aromatics compounds where detected in very low concentrations as compared to other classes (0.76% &0.74%., respectively).
GC/MS analysis of the hydro-distilled oil obtained from the flowering aerial parts of A. melampodina resulted in the identification of a total of 45 components which amounted to 97.86 % of the total oil content (Table 1). Careful analysis of the GC/MS spectrum revealed marked qualitative and quantitative differences in the chemical composition between the SPME and the hydro-distilled essential oil (Figures 1&2). The hydro-distilled oil was dominated by oxygenated sesquiterpenes that accounted for 31.22 % of the total oil content. This fraction was dominated by intermedeol (11.69%), methyl-2-epilasmona (4.19%), Z-a-santalol acetate (2.11%) and eudesmo-7(11)-en-4-o (2.11%). Monoterpene hydrocarbon accounted for (29.97%). This fraction was represented bya-pinene (15.33%) followed by myrecen (5.70%) and limonene (3.18). Oxygenated monoterpenesamounted to 20.72%andborneol (7.31%) was detected as the main compound of this fraction. The hydro-distelledoil contained sesquiterpene hydrocarbons(7.28%) with b-selinene(2.06%) ,trans-b-farnesene(1.60%),trans-caryophyllene(1.59%), germacrene D(1.26%) being detected as the main contributors to this fraction. Diterpene hydrocarbon amounted to 6.39% of the total oil content and were represented bypimaradiene(3.63%),dolabradiene (2.02%).The hydro-distilled oil contained also aliphatic hydrocarbons and their derivative (1.26 %) and aromatic compounds (1.01%). The findings of the current investigation clearly indicated that the chemical composition of A.melampodina varies with the extraction method employed.[22].
Previous studies on the essential oil of A. melampodinacollected in Egypt(1989) revealed to the isolation of Sesquiterpene lactones ,Sterols,flavonoid[22]. Again in Egypt (2002 ) The essential oil of the A. melampodina was characterized by the presence of a high percentage of monoterpene hydrocarbons (49.94%) while asesquiterpene hydrocarbons and oxygenated sesquiterpenesreported only the yield (0.03%) 7.41% and 11.43% of the oil while .In addition the essential oil of A. Melampodina was showed moderate larvacidal activity (LC(50) 139.42 ppm against culexpipens [23]. Hence, the objective of the present study was undertaken to identify the chemical composition oil of Anthemismelampodina. plant collected from Tabouk.
Table 1
|
No |
Lit RI |
exp RI |
Compound |
% SPME composition |
% Distillation composition |
|
1 |
915 |
926 |
amyl acetate |
0.35 |
– |
|
2 |
939 |
935 |
a-pinene |
– |
15.33 |
|
3 |
954 |
953 |
camphene |
– |
0.33 |
|
4 |
975 |
975 |
sabinene |
– |
0.86 |
|
5 |
979 |
981/987 |
b-pinene |
35.29 |
2.15 |
|
6 |
991 |
990 |
myrecene |
– |
5.70 |
|
7 |
1003 |
1010 |
a-phellandrene |
– |
2.09 |
|
8 |
1009 |
1015 |
hexyl acetate |
0.03 |
– |
|
9 |
1025 |
1027 |
p-cymene |
– |
0.56 |
|
10 |
1029 |
1032/1027 |
limonene |
1.78 |
3.18 |
|
11 |
1037 |
1032? |
cis-ocimene |
5.23 |
– |
|
12 |
1031 |
1035 |
1,8-cineol |
– |
6.87 |
|
13 |
1050 |
1045 |
trans-ocimene |
23.96 |
– |
|
14 |
1060 |
1062 |
g-terpinene |
6.84 |
– |
|
15 |
1070 |
1074 |
cis-sabinene hydrate |
– |
0.33 |
|
16 |
1082 |
1082 |
p-tolualdehyde |
0.64 |
– |
|
17 |
1089 |
1088 |
terpinolene |
15.78 |
– |
|
18 |
1094 |
a-campholenal |
– |
0.32 |
|
|
19 |
1121 |
1105? |
endo-fenchol |
0.15 |
– |
|
20 |
1103 |
1105 |
isoamylisovalerate |
– |
0.48 |
|
21 |
1121 |
1118 |
sabina ketone |
0.15 |
– |
|
22 |
1134 |
1131 |
1-terpineol |
0.05 |
– |
|
23 |
1144 |
1145 |
cis-b-terpineol |
0.08 |
– |
|
24 |
1145 |
1151 |
trans-verbenol |
0.10 |
0.76 |
|
25 |
1162 |
1162 |
tetrahydrolavandule |
0.94 |
– |
|
26 |
1170 |
1168 |
pinocampheol |
0.30 |
– |
|
27 |
1175 |
1173 |
isopinocamphone |
0.22 |
– |
|
28 |
1169 |
1177 |
borneol |
0.10 |
7.31 |
|
29 |
1180 |
1182 |
isopinocampheol |
– |
1.46 |
|
30 |
1189 |
1190 |
a-terpineol |
0.16 |
– |
|
31 |
1196 |
1200 |
myrtenol |
0.10 |
0.50 |
|
32 |
1229 |
1227 |
Z-ocimenone |
0.07 |
– |
|
33 |
1230 |
1232 |
nerol |
– |
2.35 |
|
34 |
1245 |
1237 |
2Z-hexenyl isovalerate |
– |
0.79 |
|
35 |
1239 |
1238 |
isoborneolformate |
0.42 |
– |
|
36 |
1249 |
1242 |
perilla ketone |
0.22 |
– |
|
37 |
1253 |
1251 |
pipertinoe |
0.09 |
– |
|
38 |
1264 |
1259 |
2E-deceneal |
0.03 |
– |
|
39 |
1272 |
1284/1286 |
perilla aldehyde |
0.16 |
0.34 |
|
40 |
1298 |
1296 |
geranylformate |
1.65 |
– |
|
41 |
1354 |
1350 |
2-phenylethyl propanoate |
0.11 |
– |
|
42 |
1393 |
1399 |
Z-jasmone |
– |
0.81 |
|
43 |
1419 |
1423 |
trans-caryophyllene |
– |
1.59 |
|
44 |
1457 |
1455 |
trans-b-farnesene |
– |
1.60 |
|
45 |
1432 |
1447 |
b-copene |
0.06 |
– |
|
46 |
1457 |
1454 |
a- patchoulene |
0.07 |
– |
|
47 |
1460 |
1461 |
all-aromadendrene |
0.14 |
– |
|
48 |
1485 |
1485 |
germacrene D |
– |
1.26 |
|
49 |
1493 |
1493 |
guaiene |
0.58 |
– |
|
50 |
1490 |
1492 |
b-selinene |
– |
2.06 |
|
51 |
1500 |
1500 |
bicyclogermacrene |
– |
0.44 |
|
52 |
1514 |
1517 |
g-cadinene |
– |
0.33 |
|
53 |
1523 |
1527 |
b-sesquiphellandrene |
1.23 |
– |
|
54 |
1531 |
1531 |
trans-g-bisabolene |
0.04 |
– |
|
55 |
1536 |
1535 |
sliphiperfol-5-en-3-ol B |
0.07 |
– |
|
56 |
1550 |
1554 |
elemol |
– |
1.05 |
|
57 |
1561 |
1558 |
germacrene D |
0.31 |
– |
|
58 |
1569 |
1566 |
longipinanol |
0.42 |
– |
|
59 |
1585 |
1573 |
globulol |
0.22 |
– |
|
60 |
1567 |
1578 |
3Z-hexenyl benzoate |
– |
0.45 |
|
61 |
1578 |
1582 |
spathulenol |
– |
2.51 |
|
62 |
1583 |
1587 |
caryophyllene oxide |
– |
0.86 |
| 63 |
1601 |
1591 |
guaiol |
0.06 |
– |
|
64 |
1596 |
carotol |
0.06 |
– |
|
|
65 |
1637 |
1634 |
gossonorol |
– |
1.11 |
|
66 |
1632 |
1638 |
g-eudesmol |
– |
1.79 |
|
67 |
1640 |
1648 |
tau-cadinol |
– |
3.00 |
|
68 |
1667 |
1662 |
intermedeol |
– |
11.69 |
|
69 |
1679 |
1681 |
methyl-z-epijasmonate |
– |
4.19 |
|
70 |
1685 |
1689 |
5-neocedranol |
– |
0.58 |
|
71 |
1700 |
1692 |
eudesm-7(11)-en-4-ol |
– |
1.03 |
|
72 |
1779 |
1175 |
Z-a-santalol acetate |
– |
2.11 |
|
73 |
1807 |
1818 |
nootkatone |
– |
1.30 |
|
74 |
1906 |
1909 |
isopimara-9(11),15-diene |
– |
0.74 |
|
75 |
1950 |
1951 |
pimaradiene |
– |
3.63 |
|
76 |
1696 |
1958 |
dolabradiene |
– |
2.02 |
| monoterpene hydrocarbons |
88.89 |
29.97 |
|||
| oxygenated monoterpenes |
4.94 |
20.72 |
|||
| sesquiterpene hydrocarbons |
2.51 |
7.28 |
|||
| oxygenated sesquiterpenes |
0.76 |
31.22 |
|||
| diterpene hydrocarbons |
0.00 |
6.39 |
|||
| aromatics |
0.74 |
1.01 |
|||
| aliphatic hydrocarbons and their derivatives |
0.40 |
1.267 |
|||
| total |
98.25 |
97.86 |
|||
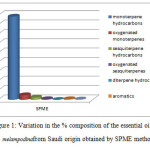 |
Figure 1: Variation in the % composition of the essential oil of A. melampodinafrom Saudi origin obtained by SPME method. Click here to View figure |
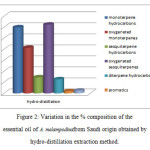 |
Figure 2: Variation in the % composition of the essential oil of A. melampodinafrom Saudi origin obtained by hydro-distillation extraction method. Click here to View figure |
References
- Bremer, K.; Humphries,C.J.;Bull. Nat. Hist. Mus. Lond. (Bot.) 1993, 23, 71.
- Abdel Ghafoor., Pak. J. Bot., Special issue (S.I. Ali Festscherift), 2010,42, 79.
- Javidnia, K.; Miri, R.;Kamalinejad, M.;Sarkarzadeh, H. Jamalian, A. FlavourFragr. J. 2004, 19, 213–216
- Kurtulmus, A.;Fafal, T.;Mert,T.;Saglam, H.;Kivcak, B.;Ozturk,T.;Demirci, B.; Baser, K. H. C.Chemistry of Natural Compounds. 2009,45(6), 900–904.
- Pavlović, M.;Lakušić, D.;Kovačević, N.;Tzakouc, O.;Couladisc, M. Chemistry & Biodiversity, 2010, 7, 1231–1244.
- Uzela, A.; Guvensena, A.; Cetinb, E. Journal of Ethnopharmacology, 2004, 95, 151–154.
- Turland, N.J. Willdenowia, 2008, 38, 61–69.
- Kilica, O.; Kocaka, A. Bagcib, E.Verlag der Zeitschriftfür Naturforschung, Tübingen, 2011, 6c, 535–540.
- Javidnia, K.; Miri, R.; Kamalinejad, M.; Sarkarzadeh, H.; Jamalian, A. FlavourFragr. J. 2004, 19, 213–216
- Baytop, T. Therapy with Medicinal Plants in Turkey (past and present), Publication of Istanbul University, (first edition), Istanbul, 1984.
- Mann, C.;Staba, E.J. The Chemistry, Pharmacology, and Commercial Formulations of Chamomille,J.of Ethnopharmacolog,2004, 95(3-4), 151–154
- Craker, L.E.; Simon J.E. (eds., Herbs, Spices, and Medicinal Plants: Recent Advances in Botany, Horticulture,and Pharmacology, 1, Oryx Press Phoenix, AZ, 1986.
- Rudzki, E.; Jalblonska, S. J. Dermatol. Treat., 2000, 11, 161.
- Bulatovic, V.M.; Menkovic, N. R.; Nebojsa, R.; Vajs, V.E.; Milosavljevic, S.M.; Slobodan, M.; Djokovic, D.D. J. Essent. Oil Res., 1998, 10, 223.
- Vajs, V.; Todorovic, N.;Bulatovic, V.; Menkovic, N.;Macura, S.;Juranic, N.;Milosavljevic,S. Phytochemistry, 1999, 50,287.
- Bruno, M.; Bondi, M.L.;Vassallo, N.;Gedris, T.E.;Herz, W. Phytochemistry, 1997, 45, 375.
- Bruno, M.; Maggio, A.; Arnold, N.A.; Diaz, J.D.;Herz, W. Phytochemistry, 1998, 46, 1739.
- Juranic, M.;Juranic, N.;Milosavljevic, S. Phytochemistry, 2000, 54, 625.
- Bruno, M.; Rosselli, S.; Bondi, T.E.; Herz, G.; Herz, W. Biochem. Syst. Ecol.,2002,30, 891.
- Williams, C.A.;Greenham, J.;Harborne, J.B. Biochem. Syst. Ecol., 2001, 29, 929.
- Chaudhary, S. Flora of the Kingdom of Saudi Arabia, 2000, Vol. 2 (part 3), Ministry of Agri. & Water, Riyadh.
- Quarenghi M.V.;Tereschuk M.L.;Baigori M.D.;Abdala L.R. Phytotherapy Res., 2002,16, 183.

This work is licensed under a Creative Commons Attribution 4.0 International License.









