Magnetic Ionic Liquid [bmim][FeCl4] as an Efficient Catalyst for the Synthesis of 2-Aryl Benzimidazoles and 2-Aryl Benzothiazoles Derivatives
Soheil Sayyahi1,2*, Sara Shabani3, Sara Ghasemi2, Atena Azin2, Seyyed Morteza Hasani1
1SAMA Technical and Vocational Training College, Mahshahr Branch, Islamic Azad University, Mahshahr,Iran.
2Department of Chemistry, Mahshahr Branch, Islamic Azad University, Mahshahr, Iran.
3Department of Chemistry, Omidieh Branch, Islamic Azad University, Omidieh, Iran.
Correspondence Author Email: sayyahi.soheil@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310359
Article Received on :
Article Accepted on :
Article Published : 29 Jul 2015
The magnetic ionic liquid (MIL) 1-butyl-3-methylimidazolium tetrachloro ferrate(III) ([bmim][FeCl4]) sufficiently catalyzes the one-pot condensation of 1,2 diaminobenzene or 2-aminobenzenethiol with different aromatic aldehydes producing benzimidazoles and benzothiazoles drivatives, respectively. The MIL showed high performance resulting great yields with appropriate reaction time.
KEYWORDS:Magnetic ionic liquid; Multicomponent Reactions; Banzimidazoles; Banzothiazoles
Download this article as:| Copy the following to cite this article: Sayyahi S, Shabani S, Ghasemi S, Azin A, Hasani S. M. Magnetic Ionic Liquid [bmim][FeCl4] as an Efficient Catalyst for the Synthesis of 2-Aryl Benzimidazoles and 2-Aryl Benzothiazoles Derivatives. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Sayyahi S, Shabani S, Ghasemi S, Azin A, Hasani S. M. Magnetic Ionic Liquid [bmim][FeCl4] as an Efficient Catalyst for the Synthesis of 2-Aryl Benzimidazoles and 2-Aryl Benzothiazoles Derivatives.Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=10082 |
Introduction
Ionic liquids (ILs) have been applied in green catalytic technologies and have been studied widely in reactions early after their discovery because of their individual unique properties such as low vapor pressure, wide liquid range, low flammability, high conductivity, excellent stability and large electrochemical window. ILs can perform specific catalytic processes and using them usually represent the advantages of high catalytic activity and also good selectivity1,2.
Otherwise, the term “magnetic ionic liquid” (MIL) was recently proposed by Hamaguchi and co-workers in 2004 to introduce ILs with paramagnetic exclusivity3,4. The MILs are primarily based on high-spin d5 Fe(III) in the form of FeCl4– or FeBr4– with various counter cations. These MILs exhibited a strong response to magnetic fields by their high single-ion magnetic moments5. The catalytic activities of MILs have been studied in Friedel crafts acylation6, aryl grignard cross coupling of alkyl halides7, preparation of 1,2-azidoalcohols8, glycolysis of poly(ethylene terephthalate) 9, “liquid fixed-bed” catalysts in flow application10, oxidative desulfurization of fuels11 and multi-component synthesis of 1- and 5-substituted 1H-tetrazoles12, quinazolines13, 1-amidoalkyl-2-naphthols14 and Xanthenedione derivatives 15.
Basically, heteroaromatic bicycles have a wide range of applications in medicinal chemistry because of their pharmaceutical and biological activities16 which are responsible for antibacterial and antifungal activity observed for benzimidazole derivatives17. The substituted benzimidazoles such as 2-Aryl and 2-alkyl benzimidazoles deliver biological activity against several viruses such as HIV, human cytomegalovirus (HCMV)18, Herpes (HVS-1)19, and influenza20. Besides, 2-Aryl benzothiazoles are important molecules due to their use not only as medicinal agents but also as organic functional materials such as fluorescent dyes and liquid crystals21. Various derivatives of benzothiazoles are also used as radioactive amyloid imaging agents22. Thus, the synthesis of these organic compounds has received considerable attention in diverse areas of chemistry. So far, a number of various synthetic methods have been developed to uncover a variety of new reagents for the preparation of benzimidazoles and benzothiazoles. The most commonly-used synthetic approaches to produce benzimidazoles typically entail the condensation of benzene-1,2-diamine with carbonyl compounds, such as aldehydes, carboxylic acids and their derivatives23-25. In addition, there are several reports on benzimidazoles synthesis via the reductive cyclization of benzene-1,2-diamine with aldehydes26, cyclization of benzene-1,2-diamine derivatives with aryl isothiocyanates27, and Baker’s yeast reduction of 2, 4-dinitroacyl anilines28. Traditional methods for the synthesis of benzothiazoles typically involve the condensation of 2-amino thiophenols with aryl aldehydes29,30, carboxylic acids31, nitriles32, acyl chlorides33,24, alcohols34, or through Jacobson’s potassium ferricyanide mediated cyclization of thiobenzanilides35. However, most of the reported methods have several drawbacks including low yield, long reaction time, the use of expensive reagents, harsh reaction conditions, tedious workup procedures, involving more than one step in their synthesis, and co-occurrence of several side reactions36.
In this paper the catalytic activity of [bmim][FeCl4] was practically investigated for the one-pot synthesis of benzimidazoles and benzothiazoles, respectively.
Experimental
General
All compounds were purchased from Aldrich and Merck companies and used as received without further purification. Progress of the reaction was monitored by TLC on Merck DC-Alufolien plates pre-coated with silica gel F254. Melting points were recorded using a Thermo Fisher Scientific IA 9200 instrument.
Synthesis of MIL [bmim][FeCl4]
The magnetic ionic liquid was synthesized following the same way remarked in literature37. 5 mmol [bmim]Cl (0.870g) and 5 mmol FeCl3 (0.825g) were added to a round bottom flask and stirred with a magnet for 15 minutes. The resulting ionic liquid was dissolved in ethyl acetate (10 mL) and the solution was centrifuged after filtration in order to separate any possible residue of inorganic salts. Afterwards, ethylacetate was evaporated and the obtained dark brown liquid, butyl methyl imidazolium tetrachloroferrate(III) was dried under vacuum at 80 ºC overnight.
General procedure for the synthesis of benzimidazoles and benzthiazoles in the presence of [bmim][FeCl4]
A mixture of 2-aminobenzenethiol or 2-aminobenzenthiol (1 mmol), aryl aldehyde (1 mmol), [bmim][FeCl4] (0.5 mmol) and ethanol (5 mL) was placed in a round bottom flask and refluxed for the given times recorded in Table 2. After completion of the reaction observed by TLC (using n-hexane/ethylacetate (7:3) as eluent), the mixture was cooled to room temperature, the precipitate was filtered and washed with water for several times. The resultant product purified by column chromatography and characterized by comparison of their physical data with benzimidazole or benzthiazole derivatives reported in literature.
Spectral data for 2-(4-nitrophenyl)-1H-benzo[d]imidazole
1HNMR was taken on a Bruker BioSpin GmbH. (400 MHz, DMSO-d6), 2.50 (s, 1H, NH), 8.42–8.41 (d, 2H, Ar–H), 7.68–7.67 (d, 2H, Ar–H), 7.66 (s, 2H, Ar–H), 7.27–7.26 (d, 2H, Ar–H).
Results and discussions
In order to investigate the catalytic ability of the MIL, the condensation of
benzene-1,2-diamine with 4-nitrobenzaldehyde was chosen as the model reaction in different conditions. As seen in Table 1, the most appropriate condition among the candidate ones giving excellent yield in a proper time is the 7th process using ethanol as solvent under reflux condition.
Table1: The one-pot condensation reaction of benzene-1,2-diamine (1 mmol) and 4-nitrobenzaldehyde (1 mmol) under different conditions
|
Result |
Time (min) |
Temperature (ºC) |
Solvent |
Catalyst (mmol) |
Entry |
|
No Reaction |
60 |
40 |
_ |
0.5 |
1 |
|
No Reaction |
60 |
r.t |
_ |
0.5 |
2 |
|
No Reaction |
60 |
reflux |
n-hexane |
0.5 |
3 |
|
Uncompleted |
60 |
reflux |
CHCl3 |
0.5 |
4 |
|
Uncompleted |
60 |
reflux |
CH3CN |
0.5 |
5 |
|
Uncompleted |
60 |
reflux |
H2O |
0.5 |
6 |
|
Completed |
40 |
reflux |
EtOH |
0.5 |
7 |
|
Uncompleted |
60 |
reflux |
EtOH |
0.25 |
8 |
|
Uncompleted |
60 |
r.t |
EtOH |
0.5 |
9 |
Then, to study the scope and limitation of these optimized procedures a wide variety of aryl benzaldehydes were exerted and the results showed a highly effective performance of the catalyst in the preparation of 2-Aryl Benzimidazoles and 2-Aryl Benzothiazoles (Scheme 1, Table 2).
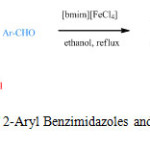 |
Scheme1: Synthesis of 2-Aryl Benzimidazoles and 2-Aryl Benzothiazoles Click here to View scheme |
![Table 2. Synthesis of 2-Aryl Benzimidazoles and 2-Aryl Benzothiazoles catalyzed by bmim[FeCl4]](http://www.orientjchem.org/wp-content/uploads/2015/07/Vol31_No3_Mag_Soh_T1-150x150.jpg) |
Table2: Synthesis of 2-Aryl Benzimidazoles and 2-Aryl Benzothiazoles catalyzed by bmim[FeCl4] Click here to View table |
It has been assumed that, magnetic ionic liquids could act as efficient catalysts due to the synergic effect of the cation and anion43. The catalytic operation of [bmim][FeCl4] is explained in the proposed mechanism (Scheme 2). Initially, the carbonyl group in aldehyde is activated by the [bmim] cation and meanwhile [FeCl4]− anion interacts with the hydrogen in X (NH2 or SH) of either benzene-1,2-diamine or 2-aminobenzenethiol. Hence, the nucleophilic attack and ring closure becomes easier to obtain the intermediate (c). Ultimately, the product is formed.
![Scheme 2. The suggested mechanism for the synthesis of 2-Aryl Benzimidazoles and 2-Aryl Benzothiazoles derivatives utilizing [bmim][FeCl4] as catalyst](http://www.orientjchem.org/wp-content/uploads/2015/07/Vol31_No3_Mag_Soh_Sch2-150x150.jpg) |
Scheme2: The suggested mechanism for the synthesis of 2-Aryl Benzimidazoles and 2-Aryl Benzothiazoles derivatives utilizing [bmim][FeCl4] as catalyst Click here to View scheme |
Conclusion
We have established an efficient method demonstrating the high catalytic activity of the magnetic ionic liquid [bmim][FeCl4] optimizing the synthesis of 2-Aryl Benzimidazole and 2-Aryl Benzothiazole derivatives, respectively. Furthermore, the synthesis procedure exhibited well to excellent yields under semi-mild conditions.
Acknowledgment
The generous support of this research by the Islamic Azad University, Mahshahr branch through grant is gratefully acknowledged.
References
- Li, H.; Bhadury, P. S.; Song, B.; Yang, S. RSC Adv. 2012, 2, 12525-12551.
- Zhang, Q.; Zhang, S.; Deng, Y. Green Chem. 2011, 13, 2619-2637.
- Hayashi, S.; Hamaguchi, H.-o. Chem. Lett. 2004, 33, 1590-1591.
- Okuno, M.; Hamaguchi, H.-o.; Hayashi, S. Appl. physics lett. 2006, 89, 132506.
- Li, M.; De Rooy, S. L.; Bwambok, D. K.; El-Zahab, B.; DiTusa, J. F.; Warner, I. M. Chem. Commun. 2009, 6922-6924.
- Valkenberg, M. H.; deCastro, C.; Hölderich, W. F. Appl. Catal., A. 2001, 215, 185-190.
- Bica, K.; Gaertner, P. Org. Lett. 2006, 8, 733-735.
- Godajdar, B. M.; Kiasat, A. R.; Hashemi, M. M. J. Mol. Liq. 2013, 183, 14-19.
- Wang, H.; Yan, R.; Li, Z.; Zhang, X.; Zhang, S. Catal. Commun. 2010, 11, 763-767.
- Misuk, V.; Breuch, D.; Löwe, H. Chem. Eng. J. 2011, 173, 536-540.
- Zhu, W.; Wu, P.; Yang, L.; Chang, Y.; Chao, Y.; Li, H.; Jiang, Y.; Jiang, W.; Xun, S. Chem. Eng. J. 2013, 229, 250-256.
- Khalafi-Nezhad, A.; Mohammadi, S. RSC Adv. 2013, 3, 4362-4371.
- Panja, S. K.; Saha, S. RSC Adv. 2013, 3, 14495-14500.
- Sayyahi, S.; Azin, A.; Saghanezhad, S. J. J. Mol. Liq. 2014, 198, 30-36.
- Mombaini Godajadar, B.; Kiasat, A. R.; Ezabadi, A. Orient. J. Chem. 2015, 31, 483-487
- Hirashima, S.; Suzuki, T.; Ishida, T.; Noji, S.; Yata, S.; Ando, I.; Komatsu, M.; Ikeda, S.; Hashimoto, H. J. Med. Chem. 2006, 49, 4721-4736.
- Stefańska, J.; Gralewska, R.; Starościak, B.; Kazimierczuk, Z. Die Pharmazie 1999, 54, 879-884.
- Porcari, A. R.; Devivar, R. V.; Kucera, L. S.; Drach, J. C.; Townsend, L. B. J. Med. Chem. 1998, 41, 1252-1262.
- Migawa, M. T.; Girardet, J.-L.; Walker, J. A.; Koszalka, G. W.; Chamberlain, S. D.; Drach, J. C.; Townsend, L. B. J. Med. Chem. 1998, 41, 1242-1251.
- Tamm, I. “Ribonucleic acid synthesis and influenza virus multiplication”; Science, 1957.
- Mori, A.; Sekiguchi, A.; Masui, K.; Shimada, T.; Horie, M.; Osakada, K.; Kawamoto, M.; Ikeda, T. J. Am. Chem. Soc. 2003, 125, 1700-1701.
- Reddy, P. V. G.; Lin, Y.-W.; Chang, H.-T. Arkivoc 2007, 16, 113-122.
- Heravi, M. M.; Tajbakhsh, M.; Ahmadi, A. N.; Mohajerani, B. Monatsh. Chem. 2006, 137, 175-179.
- Nadaf, R.; Siddiqui, S.; Daniel, T.; Lahoti, R.; Srinivasan, K. J. Mol. Catal. A: Chem. 2004, 214, 155-160.
- Niknam, K.; Fatehi-Raviz, A. J. Iran. Chem.Soc. 2007, 4, 438-443.
- Yang, D.; Fokas, D.; Li, J.; Yu, L.; Baldino, C. M. Synthesis 2005, 2005, 47-56.
- Su, Y.-S.; Sun, C.-M. Synlett 2005, 2005, 1243-1246.
- Olguin, L. F.; Jiménez-Estrada, M.; Barzana, E.; Navarro-Ocaña, A. Synlett 2005, 2005, 340-342.
- Riadi, Y.; Mamouni, R.; Azzalou, R.; Haddad, M. E.; Routier, S.; Guillaumet, G.; Lazar, S. Tetrahedron Lett. 2011, 52, 3492-3495.
- Itoh, T.; Nagata, K.; Ishikawa, H.; Ohsawa, A. Heterocycles 2004, 63, 2769-2783.
- Sharghi, H.; Asemani, O.; Khalifeh, R. Synth. Commun. 2008, 38, 1128-1136.
- Hein, D.; Alheim, R. J.; Leavitt, J. J. Am. Chem. Soc. 1957, 79, 427-429.
- Rudrawar, S.; Kondaskar, A.; Chakraborti, A. K. Synthesis 2005, 2521-2526.
- Raghavendra, G. M.; Ramesha, A. B.; Revanna, C. N.; Nandeesh, K. N.; Mantelingu, K.; Rangappa, K. S. Tetrahedron Lett. 2011, 52, 5571-5574.
- Humeník, M.; Kutschy, P.; Valková, K.; Horváth, B.; Kováčik, V.; Bekešová, S. Collect. Czech. Chem. Commun. 2005, 70, 72-84.
- Khazaei, A.; Zolfigol, M.; Moosavi-Zare, A.; Zare, A.; Ghaemi, E.; Khakyzadeh, V.; Asgari, Z.; Hasaninejad, A. Sci. Iran. 2011, 18, 1365-1371.
- Dyson, P.; Grossel, M.; Williams, D.; White, A. P. J. Chem. Soc., Dalton Trans. 1997, 3465-3469.
- Bahrami, K.; Khodaei, M. M.; Nejati, A. Green Chem. 2010, 12, 1237-1241.
- Banerjee, S.; Payra, S.; Saha, A.; Sereda, G. Tetrahedron Lett. 2014, 55, 5515-5520.
- Gadekar, L. S.; Arbad, B. R.; Lande, M. K. Chin. Chem. Lett. 2010, 21, 1053-1056.
- Matloubi Moghaddam, F.; Rezanejade Bardajee, G.; Ismaili, H.; Maryam Dokht Taimoory, S. Synth. Commun. 2006, 36, 2543-2548.
- Teimouri, A.; Chermahini, A. N.; Salavati, H.; Ghorbanian, L. J. Mol. Catal. A: Chem. 2013, 373, 38-45.
- Kodomari, M.; Tamaru, Y.; Aoyama, T. Synth. Commun. 2004, 34, 3029-3036.
- Wang, H.; Yan, R.; Li, Z.; Zhang, X.; Zhang, S. Catal. Commun. 2010, 11, 763-767.

This work is licensed under a Creative Commons Attribution 4.0 International License.









