(Caffeine)(tetrahydroborato)zinc Complex [Zn(BH4)2(caf)]: A New Stable and Efficient Reducing Agent
Fatemeh Abdollahpour and Davood Setamdideh*
Department of Chemistry, Mahabad Branch, Islamic Azad University, Mahabad, Iran. Correspondence Author Email: davood.setamdideh@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/310361
Article Received on :
Article Accepted on :
Article Published : 21 Aug 2015
In this context, (Caffeine)(tetrahydroborato)zinc complex as white stable reducing agents [Zn(BH4)2(caf)], has been prepared by complexation of oneequimolar amounts of zinc tetrahydroborate and one equimolar amounts of caffeine at room temperature. Also, [Zn(BH4)2(caf)]has been used for reduce of a variety of carbonyl compounds such as aldehydes, ketones, α, β-unsaturated carbonyl compounds, acyloins and a-diketones to their corresponding alcohols in excellent yields (90-95%). The reduction reactions have been completed in appropriate times (30-90 min) by using of 0.5-1 equivalents of [Zn(BH4)2(caf)] in CH3CN at room temperature or under reflux conditions.
KEYWORDS:Zn(BH4)2; Caffeine; Reduction; Carbonyl Compounds
Download this article as:| Copy the following to cite this article: Abdollahpour F, Setamdideh D. (Caffeine)(tetrahydroborato)zinc Complex [Zn(BH4)2(caf)]: A New Stable and Efficient Reducing Agent.HCl and Oxalic Acid. Orient J Chem 2015;31(3). |
| Copy the following to cite this URL: Abdollahpour F, Setamdideh D. (Caffeine)(tetrahydroborato)zinc Complex [Zn(BH4)2(caf)]: A New Stable and Efficient Reducing Agent.HCl and Oxalic Acid. Orient J Chem 2015;31(3). Available from: http://www.orientjchem.org/?p=10413 |
Introduction
Zn(BH4)2 is the modified borohydride agents which has better solubility in aprotic solvents, so it use and application is interesting in organic synthesis. Zinc borohydride is unique because of the coordination ability of Zn2+ which is imparting selectivity in hydride-transferring reactions.Zinc borohydride is moderately stable in ethereal solution and has many applications in organic synthesis1. Zinc borohydride as a nonconventional hydride transferring agent has been reported as an efficient chemo-, regio- and stereoselective reducing agent in several complex substrates. It can be used in a range of aprotic solvents such as, THF, Et2O and DME2.Several Combination reducing systems of Zn(BH4)2 such as Zn(BH4)2/TMEDA3a, Zn(BH4)2/Me3SiCl3b, Zn(BH4)2/TFA/DME3c, Zn(BH4)2/H2O3d, Zn(BH4)2/Al2O33e, Zn(BH4)2/C 3f, Zn(BH4)2/2NaCl3g, Zn(BH4)2/U.S.3h, and Zn(BH4)2/ZrCl43iare interesting and have been used for different reduction purposes.However, zinc tetrahydroborate has been used less than regular reducing agents in laboratory for the reduction of organic compounds, probably because of non-availability as a commercial reagent, being freshly prepared solution just prior to use and limitation to handling and storage. So, Zn(BH4)2, has been modified as stable complexes such as [Zn(BH4)2(dabco)]4,[Zn(BH4)2(pyz)]n5, [Zn(BH4)2(PPh3)] &[Zn(BH4)2(PPh3)2]6, [Zn(BH4)2(bpy)]7, [Zn(BH4)2(py)]8, [Zn(BH4)2XP4]9, [Zn(BH4)2(nmi)]10a and [Zn(BH4)2(nic)]10b. In continuation of our interest for preparation of new modified tetrahydroborates, we now wish to report the preparation of new stable ligand-zinc tetrahydroborate such as (caffeine)(tetrahydroborato)zinc complex, [Zn(BH4)2(caf)] and its reducing ability in the reduction of carbonyl compounds such as aldehydes, ketones and acyloins, a-diketones to their corresponding alcohols.
Results and Discussions
In order to determine the most appropriate reaction conditions, we first examined the reduction of benzaldehyde as a model compound as shown in Table 1. Among the tested different solvents benzaldehyde reduction was better in CH3CN. The optimization study showed that using 0.5 molar equivalents of [Zn(BH4)2(caf)] in CH3CN (3 mL) is the best conditions (Table 1, entry 9).
Table 1: Optimization of the Reduction of Benzaldehyde and Acetophenone to their Corresponding Alcohols with [Zn(BH4)2(caf)] at Room Temperature and Reflux Conditions.
| Table 1 Optimization of the Reduction of Benzaldehyde and Acetophenone to their Corresponding Alcohols with [Zn(BH4)2(caf)] at Room Temperature and Reflux Conditions. | |||||
|
Entry |
Substrate |
Molar Ratio Substrate[Zn(BH4)2(caf)] |
Solvent |
Time/min |
Conversiona/% |
|
1 |
PhCHO |
1:1 |
solvent free |
60 |
100< |
|
2 |
PhCHO |
1:1 |
n-hexane |
60 |
100< |
|
3 |
PhCHO |
1:1 |
CHCl3 |
60 |
100< |
|
4 |
PhCHO |
1:1 |
CH2Cl2 |
60 |
100< |
|
5 |
PhCHO |
1:1 |
Et2O |
60 |
100< |
|
6 |
PhCHO |
1:1 |
DME |
60 |
100< |
|
7 |
PhCHO |
1:1 |
CH3CN |
20 |
100 |
|
8 |
PhCHO |
1:1 |
THF |
40 |
100 |
|
9 |
PhCHO |
1:0.5 |
CH3CN |
30 |
100 |
|
10 |
PhCHO |
1:0.5 |
THF |
60 |
100 |
|
11 |
PhCHO |
1:0.25 |
CH3CN |
60 |
100< |
|
12 |
PhCHO |
1:0.5 |
CH3OH |
60 |
100< |
|
13 |
PhCHO |
1:0.5 |
C2H5OH |
60 |
100< |
|
14 |
PhCOCH3 |
1:0.5 |
CH3CN |
60 |
100< |
|
15 |
PhCOCH3 |
1:1 |
CH3CN |
60 |
100< |
|
16 |
PhCOCH3 |
1:2 |
CH3CN |
80 |
100 |
|
17 |
PhCOCH3 |
1:2 |
THF |
160 |
100 |
|
18b |
PhCOCH3 |
1:1 |
CH3CN |
60 |
100 |
| a Completion of reactions was monitored by TLC (eluent; Hexane/EtOAc: 10/1), bThe reaction has been carried out under reflux conditions.
|
|||||
This procedure was also applied for the reduction of various aliphatic and aromatic aldehydes (Table 2, entries 1-9). All reductions were completed within 20-50 min by 0.5 molar equivalents of [Zn(BH4)2(caf)] in excellent yields of products(90-95%).
Table 2: Reduction of a Variety of Carbonyl Compounds such as Aldehydes (entries 1-9), Ketones (entries 10-14), a-diketones (15-16), Acyloins (entriy 17) and α, β-unsaturated carbonyl Compounds (entries 18-20) to their Corresponding Alcohols with [Zn(BH4)2(caf)] as Reducing Agent in CH3CN.
| Table 2. Reduction of a Variety of Carbonyl Compounds such as Aldehydes (entries 1-9), Ketones (entries 10-14), a-diketones (15-16), Acyloins (entriy 17) and α, β-unsaturated carbonyl Compounds (entries 18-20) to their Corresponding Alcohols with [Zn(BH4)2(caf)] as Reducing Agent in CH3CN. | |||||
|
Entry |
Substrate |
Product |
Molar Ratio Substrate/[Zn(BH4)2(caf)] |
Time/min |
Yieldc/% |
|
1a |
benzaldehyde |
benzyl alcohol |
1:0.5 |
30 |
91 |
|
2a |
4-chlorobenzaldehyde |
4-chlorobenzyl alcohol |
1:0.5 |
30 |
92 |
|
3a |
4-bromobenzaldehyde |
4-bromobenzyl alcohol |
1:0.5 |
30 |
95 |
|
4a |
2,4-dichlorobenzaldehyde |
2,4-dichlorobenzyl alcohol |
1:0.5 |
30 |
94 |
|
5a |
4-methylbenzaldehyde |
4-methylbenzyl alcohol |
1:0.5 |
40 |
93 |
|
6a |
4-methoxybenzaldehyde |
4-methoxybenzyl alcohol |
1:0.5 |
40 |
92 |
|
7a |
2-methoxybenzaldehyde |
2-methoxybenzyl alcohol |
1:0.5 |
50 |
94 |
|
8a |
3-methylbenzaldehyde |
3-methylbenzyl alcohol |
1:0.5 |
35 |
92 |
|
9a |
4-nitrobenzaldehyde |
4-nitrobenzyl alcohol |
1:0.5 |
20 |
90 |
|
10b |
acetophenone |
1-phenylethanol |
1:1 |
60 |
96 |
|
11b |
benzophenone |
diphenylmethanol |
1:1 |
90 |
96 |
|
12b |
9H-fluoren-9-one |
9H-fluoren-9-ol |
1:1 |
90 |
95 |
|
13b |
cyclohexanone |
cyclohexanol |
1:1 |
50 |
95 |
|
14b |
4-phenylcyclohexanone |
4-phenylcyclohexanol |
1:1 |
50 |
98 |
|
15b |
benzil |
1,2-diphenyl ethane-1,2-diol |
1:1 |
40 |
93 |
|
16b |
1,2-bis(4-methoxyphenyl) ethane-1,2-dione |
1,2-bis(4-methoxyphenyl) ethane-1,2-diol |
1:1 |
60 |
94 |
|
17b |
benzoin |
1,2-diphenyl ethane-1,2-diol |
1:1 |
40 |
92 |
|
18a |
cinnamaldehyde |
3-phenyl-2-propen-1-ol |
1:1 |
30 |
95 |
|
19b |
benzylideneacetone |
Phenyl-3-butene-2-ol |
1:1 |
60 |
95 |
|
20b |
chalcone |
4-phenyl-3-butene-2-ol |
1:1 |
60 |
96 |
| a The reactions have been carried out at room temperature. b The reactions have been carried out under reflux conditions.c Yields refer to isolated pure products.
|
|||||
Our next attempt was the reduction of ketones. We optimized the reaction conditions with acetophenone as model compound (Table 1, entries 14-18). Due to the lower reactivity of ketones relative to aldehydes, the reduction require a higher molar amounts of [Zn(BH4)2(caf)]. The reduction reactions were carried out with 1 molar equivalents of [Zn(BH4)2(caf)] at reflux conditions in CH3CN. All reductions were completed within 40-90 min with high to excellent yields of products (92-98%) as shown in Table 2 (entries 10-17).
We also investigated the potential of the 1,2-reduction of α,β-unsaturated aldehydes and ketones with [Zn(BH4)2(caf)]. The reduction of cinnamaldehyde with 0.5 molar equivalents of the [Zn(BH4)2(caf)] exclusivity afforded the 1,2-reduction product after 30 min at room temperature in CH3CN. In this reaction, cinnamyl alcohol was obtained in 95% yield (Table 2, entry 18). Under this protocol, reduction of conjugated ketones such as benzylidenacetone (Table 2, entry 19) and chalcone (Table 2, entry 20) were achieved efficiently with 1 molar equivalents of [Zn(BH4)2(caf)] at reflux condotions in CH3CN in excellent yields (95-96%).
The efficiency of [Zn(BH4)2(caf)] has been compared with other reported reducing systems (Table 3). In all cases[Zn(BH4)2(caf)] has a good potential for the reduction of organic carbonyl compounds.
Table 3: Comparison of the Reduction of Aldehydes and Ketones by [Zn(BH4)2(caf)] in CH3CN with other Reported Reducing Agents.
|
Table 3. Comparison of the Reduction of Aldehydes and Ketones by [Zn(BH4)2(caf)] in CH3CN with other Reported Reducing Agents. |
|||||||||
|
Molar Ratio (Reagent./Substrate), Time/h |
Reducing Systems |
Entry |
|||||||
|
|
Benzoin |
9H-fluoren-9-one |
Cyclohexanone |
Benzophenone |
Acetophenone |
Benzaldehyde |
|||
|
|
1, 1 |
1 , 1.5 |
1, 0.5 |
1, 1.5 |
1, 1 |
0.5, 0.5 |
[Zn(BH4)2(caf)] |
1 |
|
|
|
1, 0.17 |
1.5, 2.3 |
– |
1.5, 8.5 |
1.2, 5.4 |
0.75, 0.7 |
[Zn(BH4)2(dabco)] |
64 |
|
|
– |
2, 0.5 |
2, 1 |
– |
2, 1.25 |
– |
[Zn(BH4)2(Ph3P)] |
75 |
||
|
0.5, 0.08 |
1, 1.5 |
0.5, 0.15 |
1, 0.75 |
0.35, 0.17 |
0.25, 0.2 |
[Zn(BH4)2(bpy)] |
87 |
||
|
0.5, 0.5 |
2, 5.3 |
2, 2 |
2, 4.3 |
2, 2 |
1, 0.5 |
[Zn(BH4)2(py)] |
98 |
||
|
3, 5 |
– |
4, 18 |
– |
4, 30 |
1, 2.5 |
[Zn(BH4)2(pyz)n] |
105 |
||
|
– |
1.6, 18 |
1, 1 |
– |
1, Im |
1, Im |
[Zn(BH4)2(nmi)] |
1110a |
||
|
– |
– |
– |
2, 21.5 |
2, 0.8 |
1, 0.25 |
[Zn(BH4)2(nic)] |
1210b |
||
|
– |
– |
2, 24 |
2, 48 |
2, 15 |
1, 8 |
[Zn(BH4)2XP4] |
139 |
||
The stability and shelf-life [Zn(BH4)2(caf)] was investigated by comparing the reduction reactions of benzaldehye as model compound under different conditions. Experiments show in adverse conditions, this reducing system is stable and reducing power remains significantly.
Experimental
All substrates and reagents were purchased from commercially sources with the best quality and used without further purification. IR and 1H NMR spectra were recorded on PerkinElmer FT-IR RXI and 300 MHz Bruker spectrometers, respectively. The products were characterized by their 1H NMR or IR spectra and comparison with authentic samples (melting or boiling points). Organic layers were dried over anhydrous sodium sulfate. All yields referred to isolated pure products. 1H NMR &TLC was applied for the purity determination of substrates, products and reaction monitoring over silica gel 60 F254 aluminum sheet.
Preparation of (Caffeine)(tetrahydroborato)zinc Complex;[Zn(BH4)2(caf)]:
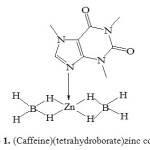
An ethereal solution of Zn(BH4)2 (0.16 M, 250 mL) was prepared from ZnCl2(5.452 g, 0.04 mol) and NaBH4 (3.177 g, 0.084 mol)according to an available procedure in the literature [12c]. Then, caffeine (7.76 g, 0.04 mol) in ether (50 mL) was added dropwise to the ethereal solution of Zn(BH4)2 and stirred for 30 min. Evaporation of the solvent under vacuum at room temperature gave [Zn(BH4)2(caf)] as a withe powder in a quantitative yield (9.16 g, 95%). Found: Zn: 22.25 %, B: 7.2 %. Calculated for C8H18B2N4O2Zn, Zn: 22.61 %, B: 7.47%. Scheme 1.
 |
Scheme 1 Click here to View scheme |
Reduction of Benzaldehyde to Benzyl alcohol with [Zn(BH4)2(caf)], A Typical Procedure:
In a round-bottomed flask (10 mL), equipped with a magnetic stirrer, a solution of banzaldehye (0.106 g, 1mmol) in CH3CN (3 mL) was prepared. The complex reducing agent (0.144 g, 0.5mmol) was then added as a solid and the mixture was stirred at room temperature. TLC monitored the progress of the reaction (eluent; CCl4/Et2O : 5/2). After completion of the reaction in 30 min, a solution of 5% HCl (5 mL) was added to the reaction mixture and stirred for 10 min. The mixture was extracted with CH2Cl2 (3 × 10 mL) and dried over the anhydrous sodium sulfate. Evaporation of the solvent and short column chromatography of the resulting crude material over silica gel by eluent of CCl4/Et2O : 5/2 afforded the pure liquid benzyl alcohol (0.097 g, 91% yield, table 1).
Conclusion
In this investigation, we have shown that [Zn(BH4)2(caf)] reduces a variety of carbonyl compounds to their corresponding alcohols in high to excellent yields. Reduction reactions were carried out with 0.5-1 molar equivalents of [Zn(BH4)2(caf)] at room temperature and reflux conditions in CH3CN without any other additive. In addition, regioselectivity of this system was also investigated with exclusive 1,2-reduction of conjugated carbonyl compounds to their corresponding allylic alcohols in high to excellent yields. Reduction of acyloins and α-diketones by this reducing system also produced the corresponding vicinal diols.
Acknowledgments
The authors gratefully acknowledge financial assistance by the research council of the Islamic Azad University branch of Mahabad.
References
- Narasimhan, S.; Balakumar, R. Aldrichim.Acta.1998,31, 19-26.
- (a) Ranu, B. C. Synlett 1993, 885-892. b) Ranu, B. C.; Chakraborty, R.Tetrahedron Lett. 1990, 31, 7663-7664. (c) Sarkar, D. C.; Das, A. R.;Ranu, B. C.J. Org. Chem. 1990, 55, 5799-5801.
- (a) Kotsuki, H.; Ushio, Y.; Yoshimura, N.; Ochi, M. Bull. Chem. Soc. Jpn. 1988,61, 2684-2686.(b) Kotsuki, H.; Ushio, Y.; Yoshimura, N.; Ochi, M. J. Org. Chem. 1987, 52, 2594-2596. (c) Ranu, B. C.; Das, A. R. J. Chem. Soc. Perkin Trans. 1 1992, 1561-1562. (d) Setamdideh, D.;Khezri, B.;Rahmatollahzadeh, M.;Aliporamjad, A.Asian J. Chem.2012, 8, 3591-3596.(e) Setamdideh, D.; Khezri, B.; Rahmatollahzadeh, M.; J. Serb. Chem. Soc. 2013, 78, 1-13. (f) Setamdideh, D.;Rahmatollahzadeh, M. J. Mex.Chem. Soc. 2012, 56, 169-175. (g) Setamdideh. D.; Khaledi, L. S. Afr. J. Chem.2013, 66, 150–157.(h) Fanari, S.;Setamdideh. D.; Orient. J. Chem., 2014, 30, 695-697. (i) Rasol, F.;Setamdideh. D.; Orient. J. Chem., 2013, 29, 497-499.
- Firouzabadi, H.;Adibi, M.;Zeynizadeh, B. Synth. Commun.1988, 28,1257-1273.
- Tamami, B.;Lakouraj, M. M.Synth. Commun.1995, 25, 3089-3096.
- Firouzabadi, H.; Adibi, M. Phosphorus Sulfur Silicon Relat. Elem. 1998, 142, 125-147.
- Zeynizadeh, B.Bull. Chem. Soc. Jpn. 2003,76, 317-326.
- (a) Zeynizadeh, B.;Faraji, F.Bull. Korean Chem. Soc.2003, 24, 453-459. (b) Zeynizadeh, B.;Zahmatkesh, K.J. Chin. Chem. Soc.2003, 50, 267-271. (c) Zeynizadeh, B.;Zahmatkesh, K.J. Chin. Chem. Soc.2004, 51, 801-806. (d) Zeynizadeh, B.;Zahmatkesh, K.J. Chin. Chem. Soc.2005, 52, 109-112.
- Firouzabadi, H.; Tamami, B.; Goudarzian, N.Synth. Commun.1991,21, 2275-2285.
- (a) Zeynizadeh, B.; Setamdideh, D. Asian J. Chem. 2009, 21, 3603-3610. (b) Setamdideh, D.;Rafig, M.E-J. Chem.2012, 9, 2338-2345.

This work is licensed under a Creative Commons Attribution 4.0 International License.









