The hunt for the dynamical resonances in chemical reaction dynamics: a perspective on historical advances
Yu Angyang1 and Zhonghua Yang2
1Chinese Academy of Sciences; 110016, Shenyang, PR China
2Liaoning University of Petroleum and Chemical Technology; 113001, Fushun, PR China
Corresponding author. E-mail address: xintongyang2011@163.com
DOI : http://dx.doi.org/10.13005/ojc/310231
Article Received on :
Article Accepted on :
Article Published : 25 Jun 2015
The theoretical background and basic definition of the resonances in chemical reaction dynamics have been introduced in this article. The historical breakthrough in the experimental search for the reaction resonances has been reviewed in this report, with an emphasis on the crossed molecular beam apparatus. The research of the chemical reaction resonances has attracted many scientists’ attention from 80s of last century. The chemical reaction resonances in the F+H2 reaction were firstly observed by the researchers of the Chinese Academy of Sciences in 2006. Besides, the partial wave resonances in the chemical reactions have been observed for the first time in 2010.
KEYWORDS:Reaction resonances; Crossed molecular beam; Differential cross section; Partial wave resonances
Download this article as:| Copy the following to cite this article: Angyang Yu, Yang Z. The hunt for the dynamical resonances in chemical reaction dynamics: a perspective on historical advances. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Angyang Yu, Yang Z. The hunt for the dynamical resonances in chemical reaction dynamics: a perspective on historical advances. Available from: http://www.orientjchem.org/?p=9423 |
Introduction
The fundamental essence of dynamical resonances
The concept of the transition state is one of the central themes in physical chemistry.[1,2] The transition state is located at a position between the reactants and products in the elementary chemical reaction, which is usually an energy barrier like a saddle point. The quantum resonances in the neighborhood of the transition state could influence the scattering behaviors of the reaction products, which contradicts with the previous analysis using the simple collision theory. Thus, the quantum dynamical processes in the neighborhood of the transition state play a crucial role in our understanding of the mechanism of elementary chemical reactions. The chemical reaction resonances is a special case of the quantum transition state, which controls the reaction rates, the products ration and the population of the products’ quantum states in the chemical reaction. Without doubt, the resonance has a great impact on the chemical reactions. [3]
Reaction resonance, which is also all the trapped state or the Feshbach resonance, is specialized at the existence of the bound state or the quasi-bound state along the reaction coordinate at the repulsive potential energy surface.[4] The reactants could be trapped into the potential energy surface, forming the quasi-bound complex corresponding to a particular quantum state. There will be a time delay which is determined by the formation of reaction products from the quasi-bound complex in the scattering processes.
As we know, a molecule is composed of many atoms through the dispersion interactions. A bound system with N atoms could have 3N-6 different vibrational modes. All the atoms could vibrate with the same frequency in the center of mass frame. Generally speaking, different atoms have different vibrational amplitudes. Only one vibrational mode moves along the reaction coordinate, which connects the reactants and the products. In addition, other vibrational modes don’t couple with the reaction coordinate and the energies of these vibrational modes could not encompass the reaction barrier. However, there is an energy redistribution process in the system, which makes the vibrational motion move along the reaction coordinate and departs the products in the reaction collision eventually. The essence of this motion is the reaction resonance, which is also regarded as the dynamical resonance because of its dynamic nature. The significance of the reaction resonance lies in the accurate description of the interaction potential in the neighborhood of the transition state region. The trivial change in the transition state region of the potential energy surface could have a great influence on the resonance effects on reaction scattering processes. [5]
The brilliant experimental research for the chemical reaction resonance states
The reaction resonance state is an instantly existing transition state of which the lifetime of the transition state is usually in the timescale of fem to second. Additionally, the molecular complex in the transition state region has a sophisticated quantum state distribution, therefore, the experimental observation of the resonance state is a very challenging work in physical chemistry. The elementary chemical reaction F+H2 serves as the classical system of the research of resonance state, which is also the main pumping step in the formation of HF chemical laser. The reaction resonance in F+H2 has been firstly predicted through theoretical calculations in 70s of last century. The resonances in chemical reactions have drawn a lot of attention in 80s of last century, however, subsequent research concerning the quantum scattering did not support the dynamical resonances observed in the experiment despite many experimental attempts in the subsequent 20 years.
The reaction resonances in the F+H2 reaction were not observed because of some unresolved issues in the experiment. It should be noted that a universal crossed molecular beam experiment was performed in order to observe the possible dynamical resonance.[6] This state-of-art experimental work about the F+H2 reaction serves as a textbook example to observe the center of mass translational speed and angular distribution of products’ vibrational states, as is illustrated in Figure 1.
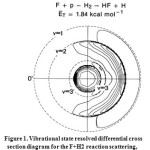 |
Figure1: Vibrational state resolved differential cross section diagram for the F+H2 reaction scattering, with HF(v=3) product forward scattered obviously. Click here to View figure |
As is shown in this figure, the HF (v=3) product is mainly forward scattered while the products HF (v=1 and 2) are backward scattered at the collision energy of 7.70Kj/mol of the F+H2 reaction. The phenomenon was attributed to the possible existence of the quantum mechanical resonance. It was believed that the HF (v=3) product is mainly resulted from the decomposition of the long-lived quasi-bound FH2 complex. That is to say, the strongly forward scattered HF (v=3) peak corresponds to the FH2 complex, which experiences some vibrational periods before dissociation. It should also be noted that the analysis of these experimental phenomena is only a prediction. Subsequent quasi-classical trajectory calculations and quantum mechanical scattering calculations did not reproduce these experimental findings.
The quantum dynamical resonances were successfully observed from the experimental research of the F+HD reaction in 2000, which is also the first experimental verification of the reaction resonances. The experimental apparatus includes the rotational molecular beam sources, the crossed molecular beam machine as well as the Doppler profile time-of-flight detection system. As is shown in figure 2, the integral cross sections observed in the experiment have shown clear fingerprints of reaction resonance. At the same time, theoretical modeling analysis has not only confirmed the existence of the reaction resonance, but also gives a detailed analysis of the resonance property.[7]
 |
Figure2: The excitation functions for F+HD→HF+D. The experimental results are shown with solid dots, the QCT stands for the quasi-classical trajectory simulations, and the QM (quantum mechanics) results include the resonance contribution and the direct reaction contribution. Click here to View figure |
As is shown in figure 2, the variation of the integral cross section with energy for the reaction channel F+HD→HF+D shows a stair-like shape, which could be reproduced by the quantum mechanical simulations while not be reproduced in the quasi-classical trajectory calculations. The theoretical analysis shows that the stair-like structure observed in the experiment is caused by the quantum resonance state, which is located near the F-H-D collinear transition state region.
The experimental detection of the reaction resonance in the F+H2 reaction is a highly difficult work in the scattering experiment. A combined theoretical and experimental investigations were performed in 2006, which observed the dynamical resonance in the F+H2 reaction for the first time.[8] The high resolution crossed molecular beam investigation of the F+H2 reaction made use of the hydrogen atom Rydberg-tagging techniques. Full quantum rovibrational-state resolved spectrum of the HF product was observed, with a clearly forward scattered HF (v=2) product peak at the collisional energy of 0.52Kj/mol. The quantum scattering theoretical analysis shows that the forward scattered peak is caused by the quantum interference effects between the ground Feshbach resonance state and the first excited Feshbach resonance state on the bound HF(v=3)-H vibrational adiabatic potential energy surface. The interferences of the two resonances contribute to the enhancement of the forward scattered peak. It is usually difficult to observe the resonances in the realm of atomic and molecular physics. The possible reason is the enormous energy levels and the dense energy intervals. Indeed, the high velocity and angular distribution resolution achieved in the experiment is very necessary in order to probe the dynamical resonances.
The observation of the reaction resonances is a new historical breakthrough in the chemical reaction dynamics, which permits the observation of the full quantum state resolution spectrum of the F+H2 system at an unprecedented level. It should be noted that the forward scattered product is an important detection method of reaction resonance, which is due to the close connection between the forward reaction scattering and the time delay of the bound transition state.
The first observation of the partial wave resonance in the chemical reaction
The observation of the dynamical phenomenon induced by the partial wave has always been a challenging topic in the field of chemical dynamics experiment. By designing the highest resolution crossed molecular beam apparatus in the world, it is feasible to observe the partial wave resonances in the chemical reaction.[9] The partial wave structure of the transition state is the determining factor which influences the elementary chemical reaction. In spite of some advances of geometric phase effects in the chemical dynamics,[10] observing the specific partial wave resonance is a big challenge in the field of chemical dynamics. The reaction resonance is a quasi-bound state with a limited lifetime in the neighborhood of the transition state region. It serves as an opportunity of observing the dynamical phenomenon of single partial wave resolution because of the different energies and lifetimes of different partial resonance states. The most promising reaction system of observing the single partial-wave resonance structure in the experiment is the F+HD→HF+D elementary chemical reaction. Since the lifetime of the resonance state in the F+HD reaction is hundreds of femtoseconds, it is possible to detect the single partial wave resonance structure. It is also necessary to improve the resolution of the crossed molecular beam apparatus in order to resolve the different partial wave resonance structure, as is shown in Figure 3. The dotted experimental results could be explained by the theoretical analysis that the three ossilatory peaks correspond to the rotational quantum state 12, 13 and 14, respectively.
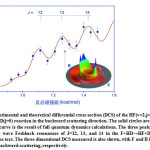 |
Figure3: Experimental and theoretical differential cross section (DCS) of the HF(v=2,j=6) product of the F(2P3/2)+HD(j=0) reaction in the backward scattering direction. The solid circles are experimental data; the red curve is the result of full quantum dynamics calculations. The three peaks are assigned to the partial wave Feshbach resonances of J=12, 13, and 14 in the F+HD→HF+D reaction, as explained in the text. The three dimensional DCS measured is also shown, with F and B indicating the forward and backward-scattering, respectively. Click here to View figure |
Conclusions
After a lot of combined research of theoretical and experimental work, we could detect and explain the variation of differential cross section with the collisional energy, which is resulted from the resonance states. The observation of the quantum resonance effects in the reaction scattering, which was predicted by the theoretical calculations and further confirmed by the experiment, prove to be a successful milestone in the history of chemical dynamics. Without doubt, the quantum theory serves as a good guide for the description of the partial wave resonance state induced differential cross section’s variation with the collisional energy. The interaction of experiment and theory has promoted the development of this series of resonance state research. The new experiment phenomena could guide the theoretical work to construct the most accurate potential energy surface. More accurate theoretical results could help discover new phenomena in the experiment and contribute to the development of the theory itself. Overall, the understanding of resonance states has lifted the chemical dynamics into a higher level.
Acknowledgements
Many thanks for the reviewer’s helpful and constructive comments.
References
- Klippenstein, S.J.; Pande, V.S.; Truhlar, D.G. J. Am. Chem. Soc. 2014, 136, 528-546
- Yu, A. Y. J. Ir. Chem. Soc. 2014, 11,593-598
- Schatz, G.C.; Bowman, J.M.; Kuppermann, A. J. Chem. Phys. 1975, 63,674-684
- Zare, R.N. Science. 2006, 311, 1383-1385
- Liu, K. Annu.Rev.Phys.Chem. 2001, 52, 139-164
- Neumark, D.M.; Wodtke, A.M.; Robinson, G.N.; Hayden, C.C.; Shobatake, K.; Sparks, R.K.; Schafer, T.P.; Lee, Y.T. J. Chem. Phys.1985, 82, 3067-3077
- Skodje,R.T.; Skouteris, D.; Manolopoulos, D.E.; Lee, S.H.; Dong, F.; Liu, K. J. Chem. Phys.2000,112,4536-4552
- Qiu, M.H.; Ren, Z.F.; Che, L.; Dai, D.X.; Harich, S.A.; Wang, X.Y.; Yang, X.M.; Xu, C.X.; Xie, D.Q.; Gustafsson, M.; Skodje, R.T.; Sun, Z.G.; Zhang, D.H. Science. 2006, 311, 1440-1443
- Dong, W.R.; Xiao, C.L.; Wang, T.; Dai, D.X.; Yang, X.M.; Zhang, D.H.Science.2010,327,1501-1502
- Yu, A.Y. Prog. Chem. 2008, 20, 208-211

This work is licensed under a Creative Commons Attribution 4.0 International License.









