Synthesis of Novel Fused pyrimidines and imidazoles as potential analgesics from 2-Amino-4-substituted-s-triazino[1,2-a]-benzimidazoles
Said A. El-Feky1,2*, Hamdy Kh. Thabet3,4, Mahmoud M. E. Mudawi5,6
1Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Northern Border University, Rafha, 91911, P.O. 840, Saudi Arabia.
2Department of Pharmaceutical Organic Chemistry, Faculty of Pharmacy, Zagazig University, Zagazig, 44511, Egypt.
3Department of Chemistry, Faculty of Arts and Science, Northern Border University, Rafha, 91911, P.O. 840, Saudi Arabia.
4Department of Chemistry, Faculty of Science, Al-Azhar University, 11284, Nasr City, Cairo, Egypt.
5Department of Pharmacology and Toxicology, Faculty of Pharmacy, Northern Border University, Rafha, 91911, P.O. 840, Saudi Arabia.
6Department of Pharmacology, Faculty of Pharmacy, Omdurman, Islamic University, Sudan.
DOI : http://dx.doi.org/10.13005/ojc/310213
Article Received on :
Article Accepted on :
Article Published : 06 May 2015
The synthesis of novel fused pyrimidines and imidazole derivatives from 2-amino-s-triazino[1,2-a]benzimidazoles2a-e and 3a-c was successfully carried out by a ring annelation reaction in a very good yield. Compound 3c was screened for analgesic activity against acetic acid irritation and has shown protection equal to the reference drug (diclofenac sodium). The acute toxicity study revealed that compound 3c is safe up to 300 mg/kg and there is no sign and symptoms of toxicity and mortality for 72 hours.
KEYWORDS:2-Guanidinobenzimidazoles; cyclocondensation; fused pyrimidines; fused imidazoles; analgesic activity
Download this article as:| Copy the following to cite this article: El-Feky S. A, Thabet H. KH, Mudawi M. M. E. Synthesis of Novel Fused pyrimidines and imidazoles as potential analgesics from 2-Amino-4-substituted-s-triazino[1,2-a]-benzimidazoles. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: El-Feky S. A, Thabet H. KH, Mudawi M. M. E. Synthesis of Novel Fused pyrimidines and imidazoles as potential analgesics from 2-Amino-4-substituted-s-triazino[1,2-a]-benzimidazoles. Orient J Chem 2015;31(2). Available from: http://www.orientjchem.org/?p=8795 |
Introduction
Azole class of drugs, particularly fused imidazole occupy a prominent place in medicinal chemistry because of their broad spectrum of pharmacological activities such as anti-inflammatory, analgesic, anticancer, antiulcer, antimicrobial, antiviral, pesticidal and anti-arrhythmic activities1-4. Omeprazole, mebendazole and abendazole are well known drugs in the market which contain fused imidazole as active core moiety.
1,3,5-Triazine (s-triazines) derivatives, which synthesized via heterocyclic-zation of bigunaidines or their analogues using β-keto ester5 such as Tretamines, Furazil and Dioxadet, have been known as anticancer drugs6. Moreover, an anti-gastric ulcer agent that is commonly used in Japan, irsogladine (2-amino-1,3,5-triazine), was shown to possess antiangiogenic properties which result in the anticancer effect of the drug7. It was reported8 that compounds having the core structure of s-triazino[1,2-a]benzimidazole(A) with particular reference to 2-amino-4,4,7,8-tetramethyl-3,4-dihydro-s-triazino[1,2-a]benzimid-azole (B), have demons-trated inhibitory activity against the plasmodial DHER.
 |
Structure A,B |
Pyrimidines and fused pyrimidines, being an integral part of DNA and RNA, play an essential role in several biological processes. They also have considerable chemical and pharmacological importance, particularly, as nucleoside antibiotics, antibacterial, cardiovascular as well agrochemical and veterinary products9. Various pyrimidine derivatives showed analgesic, antiarrhythmic and anticancer activities10, as well anti-inflammatory, antiparkinsonian and androgenic anabolic activities11, anticonvulsant12, and antihypertensive activities13.
Encouraged by the above observations and in continuation of our work for the syntheses of biologically active heterocyclic lead compounds2,3,14,15, a new series of fused pyrimidines(8, 13, 15a-f, 19a-d) and fused imidazoles(20a,b) were synthesized with a view to explore the possibility of achieving a new class of heterocylic compounds possessing potent analgesic activity.
Resultsand Discussion
Chemistry
The reaction sequence used to synthesize the target compounds is illustrated in Schemes 1-6. The key intermediate, 2-guanidino-benzimidazole (1) was prepared by cyclocondensation of o-phenylenediamine with dicyandiamide in acidic medium16 under reflux temperature. The synthesis of 3,4-dihydro[1,3,5]triazino[1,2-a]-benzimidazole-2-amines through a base catalyzed cyclization of 2-guanidinobenz-imidazole (1) with benzaldehyde was first reported by Nagarajanet al in 197017. Using a variety of aromatic aldehydes and ketones in the presence of piperidine as a catalyst, we have prepared 4-aryl-3,4-dihydro[1,3,5]triazino[1,2-a]benzimidazole-2-amines 2a-e & 3a-c (Scheme 1); as a starting material in our study to capitalize on the biological potential of these new heterocyclic systems.
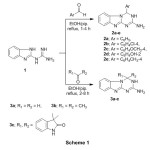 |
Scheme 1 Click here to View Scheme |
It has been found that, the treatment of 4,4-dimethyl-3,4-dihydrobenzo[4,5]-imidazo[1,2-a][1,3,5]triazin-2-amine 3b with cinnamonitrile4 in N,N-dimethylf-ormamide at reflux temperature in the presence of trimethylamine as a catalyst gave the novel 4-amino-2-(4-chlorophenyl)-6,6-dimethyl-6H-benzo[4,5]imidazo[1,2-a]-pyrimido-[2,1-d][1,3,5]triazine-3-carbonitrile 8 (Scheme 2). The two other possible structures 5&6 were excluded upon the elemental analyses and spectral data. The infrared and the 1HNMR spectra were in complete agreement with the structure of compound 8.The infrared spectra of compound 8 showed the presence of the NH2 group at 3380, 3146 cm-1 and C≡N at 2210 cm-1. The 1H NMR spectrum (DMSO-d6) revealed a singlet at 1.96 ppm due to two methyl groups and a singlet at 6.49 ppm which was assigned to the NH2 protons, in addition to the presence of aromatic protons 7.23-7.64 ppm. The reaction occurs via an initial formation of the Michael adduct A from the Michael addition of amino exocyclic in 2-aminotriazinobenz-imidazole derivative 3b to the activated double bond in compounds4. The latter adduct undergo cyclization to give the non-isolable intermediate 7 followed by aromatization via loss of H2 molecule18 to give compounds 8.
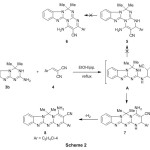 |
Scheme 2 Click here to View Scheme |
As an extension of such synthetic route, the behavior of 3b toward ethyl α-cyanocinnamate9 was also investigated. The reaction of compound 3b with ethyl α-cyanocinnamate9 in refluxing N,N-dimethylformamide in the presence of trimethyl-amine as a catalyst gave the corresponding 2-(4-chlorophenyl)-6,6-dimethyl-6H-benzo[4,5]-imidazo[1,2-a]pyrimido[2,1-d][1,3,5]triazin-4-amine 13 rather than the compound 11 (Scheme 3). Evidence for the structure of compounds 13 included the infrared spectra which revealed absorption bands for the NH2 and C=O groups and the absence of the absorption band of carbonitrile group. The infrared spectrum of compound 13 displayed the absorption bands for NH2 at 3350, 3170 cm-1, aliphatic-CH at 2970 cm-1, C=O at 1666 cm-1, C=N at 1615 cm-1. The 1HNMR spectra of the reaction product displayed the absence of the lack of signals characteristic for ethyl protons. The 1HNMR spectrum (DMSO-d6) of this revealed a singlet at 1.84 ppm assigned for two geminal methyl protons, a singlet at 5.84 assigned for methine-H, a singlet at 7.95 ppm assigned for amino group, in addition to the presence of aromatic protons at 6.76-7.61 ppm in the spectrum.
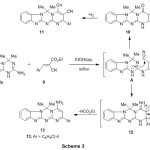 |
Scheme 3 Click here to View scheme |
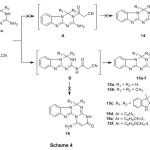
The treatment of compounds 2a-d, 3a-c with ethyl cyanoacetate in N,N-dimethylformamide at reflux temperature gave the novel 4-amino-benzo[4,5]-imidazo[1,2-a]pyrimido[2,1-d][1,3,5]triazin-2(1H)-ones 15a-f (Scheme 4). Theoretically, the cyclization reaction of 2-amino-4-aryl-3,4-dihydro[1,3,5]triazino-[1,2-a]benzimidazoles2a-d, 3a-d with ethyl cyano acetate may proceed in several ways. The most probable approaches include:
The initial attack of endocyclic N-3 of 2-amino-4-aryl-3,4-dihydro[1,3,5]triazino-[1,2-a]benzimidazoles2a-d,3a-c with ethyl cyanoacetate followed by intramolecular cyclization of the presumable intermediate A with formation of the heterocyclic system 14.
The initial attack of exocyclic amino group nitrogen of 2-amino-4-aryl-3,4-dihydro[1,3,5]triazino[1,2-a]benzimidazoles2a-d, 3a-c with ethyl cyanoacetate followed by ring closure of the presumable intermediate B to N-3 or N-1 with formation of the heterocyclic system 15 or 16, respectively..
The structures of compounds 15a-f were elucidated by the elemental analyses and spectral data. For example, the infrared spectrum of compounds 15a-f exhibited the absorption bands of NH2, NH between 3400-3110 cm-1, carbonyl group from 1690-1658 cm-1. The 1HNMR spectrum (DMSO-d6) of compound 15a revealed a singlet at 5.49 ppm assigned for N(CH2), 5.90 ppm for pyrimidine-H, 6.03 ppm for NH2, a singlet at 7.62 assigned for NH, in addition to the presence of aromatic protons at 7.13-7.45 ppm. Also, the 1HNMR spectrum of compound 15b displayed a singlet at 1.90 ppm assigned for two germinal methyl protons, a singlet at 8.69 ppm assigned ofNH, in addition to the presence of aromatic protons at 7.09-7.72 ppm.
 |
Scheme 4 Click here to View scheme |
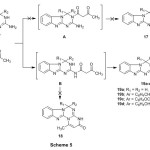
Also, the reaction of 2c-e, 3a,b with ethyl acetoacetate led to the formation of condensation product which may be formulated as 4-methyl-6-aryl-6H-benzo[4,5]-imidazo[1,2-a]pyrimido[2,1-d][1,3,5]triazin-2(1H)-ones 19a-d (Scheme 5). The other possible structure 18 was excluded according to previously reported data19. The elemental and spectral analysis of the isolated products was consistent with both structures 19a-d (cf. Experimental). The ir spectrum of compound 19b exhibited absorption bands at 3130 for NH, 2971 cm-1 for aliphatic-CH, 1689 cm-1 for carbonyl group. The 1HNMR spectrum (DMSO-d6) of compound 19b two singlets at 2.13, 2.38 ppm assigned for two methyl protons, two singlets at 5.52, 5.67 ppm assigned for pyrimdine-H, and triazine-H, respectively, a singlet at 7.87 assigned for NH, in addition to the presence of aromatic protons at 6.98-7.45 ppm in the spectrum. 13CNMR spectrum (DMSO-d6) of compound 19b displayed signal at 165.12 ppm assigned for carbonyl group, 159.31, 157.02, 150.38 ppm for C=N groups, 64.91 ppm assigned for triazine-C, and 20.63, 14.08 ppm assigned for 2 methyl carbon’s, in addition to aromatic carbons at 148.84-109.73 ppm.
 |
Scheme 5 Click here to View scheme |
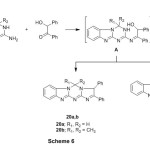
Finally, the cyclocondensation of 2-amino-4-aryl-3,4-dihydro[1,3,5]triazino-[1,2-a]benzimidazoles with benzoin gave 2,3-diphenyl-3H,5H-benzo[4,5]imidazo-[1,2-a]imidazo[2,1-d][1,3,5]triazines 20a,b andthe other possible structure 5-aryl-1,2-diphenyl-1,5-dihydrobenzo[4,5]imidazo[1,2-a]-imidazo[1,2-c][1,3,5]triazines 21a,b was excluded according to previously reported data17 (Scheme 6). The infrared spectrum of compound 20a exhibited absorption bands at 2971 cm-1 for aliphatic-CH, 1610 cm-1 for C=N. the 1HNMR spectrum (DMSO-d6) of compound 20b displayed a singlet at 1.92 ppm assigned for two geminal methyl protons, a singlet at 5.38 ppm assigned for imidazole-H, in addition to the presence of aromatic protons at 6.87-7.78 ppm.
 |
Scheme 6 Click here to View scheme |
Pharmacology
A preliminary pharmacological screening of compound 3c for analgesic activity was carried out adopting acetic acid induced writhing test20. The results were expressed as mean ± SEM and statistical comparisons were made by conducting one way ANOVA (p<0.05) (table 1).
Table 1: effect of synthesized drug on acetic acid induced writhing in mice
|
Group |
Dose |
Number of writhing(Mean ±SEM) |
Percent inhibition |
|
Group-I |
Control (10 ml/kg 2% aqueous gum) |
28.20 ± 6.20 |
– |
|
Group-II |
positive control (diclofenac sodium 20 mg/kg, oral) |
7.70 ± 2.10 *↓ |
72.70 |
|
Group-III |
Synthesized compound (20 mg/kg, oral) |
11.60 ± 4.34 *↓ |
58.87 |
|
Group-IV |
Synthesized compound (40 mg/kg, oral) |
8.10 ± 2.61 *↓ |
71.28 |
One way ANOVA (P ˂ 0.05)*↓ Significant reduction
The results from table 1 indicate that compound 3c either in oral dose 20mg/kg or oral dose 40mg/kg and the reference drug (diclofenac sodium) in oral 20mg/kg give a significant reduction in the number of writhes compared to the control which administered in oral dose 10 ml/kg in 2% aqueous gum acacia. Also, from table 1 the results of percentage inhibition indicate that there is no significance difference between the treated groups (71.28% inhibition of compound 3c in comparison to 72.70% of diclofenac sodium). These results are in accordance to the reported data21,22 that acetic acid induces pain sensation through prostaglandin (PG) biosynthesis and hence the writhing response is associated with increased level of PGE2 and PGE2α.
On the light of the above data we can surmise that compound 3c produce its analgesic effect by the interference with prostaglandin synthesis.
The acute toxicity study of compound 3c was performed on the basis of OECD/OCDE guidelines No: 42323 in a dose level of 300 mg/kg body weight, and the behavioral and physiological effects24 were recorded.
Table 2 (A): The acute oral toxicity for the control Group: 2% Aqueous gum solution
|
Death (hours) |
Physiological alterations (hours) |
Wt. (g) |
No. |
||||||||||||||||||||||
|
Decreased M.A |
Increased M.A |
Convulsions |
Resp. Arrest |
Sedation |
Hyperactivity |
Grooming |
|||||||||||||||||||
|
72 hrs |
24hrs |
1hr |
72hrs |
24hrs |
1hr |
72hrs |
24hrs |
1hr |
72hrs |
24hrs |
1hr |
72hrs |
24hrs |
1hr |
72hrs |
24hrs |
1hr |
72hrs |
24hrs |
1hr |
72hrs |
24hrs |
1hr |
||
|
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
+ |
29g |
1 |
|
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
31g |
2 |
|
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
+ |
29g |
3 |
Table 2 (B:) The acute oral toxicity for the group administered the synthesized compound (300mg\kg)
|
Death (hours) |
Physiological alterations (hours) |
Wt. (g) |
NO |
||||||||||||||||||||||
|
Decreased M.A |
Increased M.A |
Convulsions |
Resp. Arrest |
Sedation |
Hyperactivity |
Grooming |
|||||||||||||||||||
|
72 hrs |
24hrs |
1hr |
72hrs |
24hrs |
1hr |
72hrs |
24hrs |
1hr |
72hrs |
24hrs |
1hr |
72hrs |
24hrs |
1hr |
72hrs |
24hrs |
1hr |
72hrs |
24hrs |
1hr |
72hrs |
24hrs |
1hr |
||
|
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
+ |
27g |
1 |
|
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
29g |
2 |
|
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
– |
+ |
28g |
3 |
The results of table 2 (A & B) reveals that there is no toxicity observed for the compound 3c at the dose level of 300 mg/kg up to 72 hours. So, LD50 of compound 3d will be more than 300mg/kg body weight.
Conclusion
Several novel fused pyrimidines and imidazoles derived from 2-amino-s-triazino[1,2-benzimidazoles were synthesized. The preliminary pharmacological screening for analgesic activity of these compounds revealed that they possess potent analgesic activity. Compound 3c possesses a potent analgesic activity equal that of diclofenac sodium (Voltraen). These results indicate that the new compounds may represent a novel analgesic and hence they are ideally suited for further study and could be developed as lead compounds in novel class of analgesics.
Experimental
All melting points are uncorrected. IR spectra (KBr) were measured on Shimadzu 440 spectrometer, 1HNMR and 13CNMR spectra were obtained in DMSO-d6on a Varian Gemini 600 MHz spectrometer using TMS as internal standard; chemical shifts are reported as (ppm). Elemental analyses were carried out at the Department of Chemistry, Faculty of Science, King Abdul-Aziz University, Jeddah 21589, KSA. Compounds 2a-e, 3a-c were prepared according to reported procedure25. 2-Amino-3H-spiro[benzo[4,5]-imidazo[1,2-a][1,3,5]triazine-4,3′-indolin]-2′-one 3c; yield (81%); m.p: 310-312ºC; ir (potassium bromide, cm-1): 3384, 3272, 3180 (NH2/NH), 1670 (C=O), 1615 (C=N); 1H-NMR (600 MHz, DMSO-d6): δ 6.78 (s, 2H, NH2), 7.08-7.63 (m, 8H, Ar-H), 8.31, 11.66 (2s, 2H, 2NH); Anal. Calcd. for C16H12N6O: C, 63.15; H, 3.97; N, 27.62. Found: C, 63.03; H, 3.86; N, 27.45.
4-Amino-2-(4-chlorophenyl)-6,6-dimethyl-6H-benzo[4,5]imidazo[1,2-a]pyrimido-[2,1-d][1,3,5]triazine-3-carbonitrile 8.
A solution of 3b (0.01 mole), 2-(4-chlorobenzylidene)malononitrile (0.01 mole) and triethylamine (0.5 mL) in N,N-dimethylformamide (30 ml) was refluxed for 4 hours. The precipitated solid was filtered on hot and recrystallized from dioxane to give compound 8 as yellow crystals, yield (70%); m.p: 300-302ºC; ir (potassium bromide, cm-1): 3380, 3146 (NH2), 2970 (CH-aliph.), 2210 (C≡N), 1580 (C=N); 1H-NMR (600 MHz, DMSO-d6): δ 1.96 (s, 6H, 2 CH3), 7.97 (s, 2H, NH2), 7.23-7.64 (m, 8H, Ar-H); Anal. Calcd. for C21H16ClN7: C, 62.77; H, 4.01; N, 24.40. Found: C, 62.62; H, 3.92; N, 24.32.
2-(4-chlorophenyl)-6,6-dimethyl-6H-benzo[4,5]-imidazo[1,2-a]pyrimido[2,1-d]-[1,3,5]triazin-4-amine13
A solution of 3b (0.01 mole), 2-(4-chlorobenzylidene)malononitrile (0.01 mole) and triethylamine (0.5 mL) in N,N-dimethylformamide (30 ml) was refluxed for 6 hours. The solid that obtained on cooling was collected by filtration and recrystallized from acetic acid to give compound 13 as yellow crystals, yield (66%); m.p: 297-299ºC; IR (potassium bromide, cm-1): 3350, 3170 (NH2), 2970 (CH-aliph.), 1666 (C=O), 1615 (C=N); 1H-NMR (600 MHz, DMSO-d6): δ 1.84 (s, 6H, 2 CH3), 5.84 (s, 1H, CH), 6.76-7.61 (m, 8H, Ar-H), 7.95 (s, 2H, NH2); Anal. Calcd. for C20H17ClN6: C, 63.74; H, 4.55; N, 22.30. Found: C, 63.62; H, 4.42; N, 22.20.
Synthesis of Benzo[4,5]imidazo[1,2-a]pyrimido[2,1-d][1,3,5]triazin-2(1H)-ones15a-f
General procedure: A solution of 2a-d and/or 3a-c (0.01 mole), ethyl cyanoacetate (0.01 mole) in N,N-dimethylformamide (30 ml) was refluxed for 8 hours. The solid that obtained on cooling was collected by filtration and recrystallized from proper solvent to give compounds 15a-f.
4-Amino-6H-benzo[4,5]imidazo[1,2-a]pyrimido[2,1-d][1,3,5]triazin-2(1H)-one 15a.
Yield (72%); acetic acid (yellow crystals); m.p: 308-310ºC; ir (potassium bromide, cm-1): 3310, 3130 (NH2), 2876 (CH-aliph.), 1668 (C=O), 1619 (C=N); 1H-NMR (600 MHz, DMSO-d6): δ 5.49 (s, 2H, N(CH2)), 5.90 (s, 1H, CH), 6.03 (s, 2H, NH2), 7.13-7.45 (m, 4H, Ar-H), 7.62 (s, 1H, NH); Anal. Calcd. for C12H10N6O: C, 56.69; H, 3.96; N, 33.05. Found: C, 56.57; H, 4.01; N, 32.92.
4-Amino-6,6-dimethyl-6H-benzo[4,5]imidazo[1,2-a]pyrimido[2,1-d][1,3,5]triazin-2(1H)-one 15b.
Yield (78%); DMF (faint yellow crystals); m.p: 312-314ºC; ir (potassium bromide, cm-1): 3350, 3170 (NH2), 2970 (CH-aliph.), 1670 (C=O), 1615 (C=N); 1H-NMR (600 MHz, DMSO-d6): δ 1.90 (s, 6H, 2CH3), 6.47 (s, 1H, CH), 7.09-7.72 (m, 6H, Ar-H + NH2), 8.69 (s, 1H, NH), Anal. Calcd. for C14H14N6O: C, 59.56; H, 5.00; N, 29.77. Found: C, 59.44; H, 4.88; N, 29.63.
Aminospiro[benzo[4,5]imidazo[1,2-a]pyrimido[2,1-d][1,3,5]triazine-6,3′-indo-line]-2,2′(1H)-dione15c.
Yield (71%); dioxane (brown crystals); m.p: 300-301ºC; ir (potassium bromide, cm-1): 3420, 3244, 3150 (NH2/NH), 2970 (CH-aliph.), 1690, 1669 (C=O), 1608 (C=N); 1H-NMR (600 MHz, DMSO-d6): δ 6.48 (s, 1H, CH), 7.95 (s, 2H, NH2), 6.94-7.96 (m, 8H, Ar-H), 8.65 (s, 1H, NH), 10.09 (s, 1H, NH), 12.48 (s, 1H, NH); Anal. Calcd. for C19H13N7O2: C, 61.45; H, 3.53; N, 26.40. Found: C, 61.50; H, 3.47; N, 26.29.
4-Amino-6-phenyl-6H-benzo[4,5]imidazo[1,2-a]pyrimido[2,1-d][1,3,5]triazin-2(1H)-one15d.
Yield (68%); acetic acid (white crystals); m.p: 286-288ºC; ir (potassium bromide, cm-1): 3415, 3230 (NH2), 2986 (CH-aliph.), 1658 (C=O), 1620 (C=N); 1H-NMR (600 MHz, DMSO-d6): δ 5.98 (s, 1H, CH), 6.41 (s, 1H, CH), 7.21-7.48 (m, 9H, Ar-H), 7.96 (s, 2H, NH2), 12.51 (hump, 1H, NH); Anal. Calcd. for C18H14N6O: C, 65.44; H, 4.27; N, 25.44. Found: C, 65.34; H, 4.20; N, 25.31.
4-Amino-6-(2-hydroxyphenyl)-6H-benzo[4,5]imidazo[1,2-a]pyrimido[2,1-d][1,3,5]-triazin-2(1H)-one 15e.
Yield (65%); DMF (brown crystals); m.p: 302-304ºC; ir (potassium bromide, cm-1): 3328, 3220 (NH2), 2970 (CH-aliph.), 1670 (C=O), 1622 (C=N); 1H-NMR (600 MHz, DMSO-d6): δ 5.88 (s, 1H, CH), 6.23 (s, 1H, CH), 7.14-7.52 (m, 8H, Ar-H), 7.86 (s, 2H, NH2), 12.66 (hump, 2H, NH, OH); Anal. Calcd. for C18H14N6O2: C, 62.42; H, 4.07; N, 24.27. Found: C, 62.28; H, 4.00; N, 24.18.
4-Amino-6-(4-methoxyphenyl)-6H-benzo[4,5]imidazo[1,2-a]pyrimido[2,1-d][1,3,5]-triazin-2(1H)-one 15f.
Yield (69%); dioxane (white crystals); m.p: 305-306ºC; ir (potassium bromide, cm-1): 3415, 3178 (NH2), 2970 (CH-aliph.), 1671 (C=O), 1620 (C=N); 1H-NMR (600 MHz, DMSO-d6): δ 3.74 (s, 3H, OCH3), 6.08 (s, 1H, CH), 6.47 (s, 1H, CH), 6.63-7.35 (m, 10H, Ar-H+NH2), 8.22 (s, 1H, NH); Anal. Calcd. for C19H16N6O2: C, 63.33; H, 4.48; N, 23.32. Found: C, 63.40; H, 4.33; N, 23.20.
Synthesis of Benzo[4,5]imidazo[1,2-a]pyrimido[2,1-d][1,3,5]triazin-2(1H)-ones19a-e:
General procedure: A solution of 2a-d and/or 3a-c (0.01 mole), ethyl acetoacetate (0.01 mole) in N,N-dimethylformamide (30 ml) was refluxed for 6 hours. The solid that obtained on cooling was collected by filtration and recrystallized from proper solvent to give compounds 19a-d.
4-Methyl-6H-benzo[4,5]imidazo[1,2-a]pyrimido[2,1-d][1,3,5]triazin-2(1H)-one 19a.
Yield (64%); acetic acid (white crystals); m.p: 308-310ºC; ir (potassium bromide, cm-1): 3150 (NH), 2970 (CH-aliph.), 1668 (C=O), 1636 (C=N); 1H-NMR (600 MHz, DMSO-d6): δ 2.14 (s, 3H, CH3), 5.54 (s, 1H, CH), 5.94 (s, 2H, N(CH2)), 6.03 (s, 2H, NH2), 7.09-7.46 (m, 4H, Ar-H), 12.21 (s, 1H, NH); Anal. Calcd. for C13H11N5O: C, 61.65; H, 4.38; N, 27.65. Found: C, 61.49; H, 4.26; N, 27.51.
4-Methyl-6-(p-tolyl)-6H-benzo[4,5]imidazo[1,2-a]pyrimido[2,1-d][1,3,5]triazin-2(1H)-one 19b.
Yield (60%); DMF (yellow crystals); m.p: 305-307ºC; ir (potassium bromide, cm-1): 3130 (NH), 2971 (CH-aliph.), 1689 (C=O), 1645 (C=N); 1H-NMR (600 MHz, DMSO-d6): δ 2.13 (s, 3H, CH3), 2.38 (s, 3H, CH3), 5.52 (s, 1H, CH), 5.67 (s, 1H, CH), 6.98-7.45 (m, 8H, Ar-H), 7.87 (s, 1H, NH); 13C-NMR (600 MHz, DMSO-d6): δ 14.08, 20.63, 59.28, 109.73, 115.99, 120.20, 121.09, 122.22, 126.19, 126.24, 129.35, 130.95, 134.08, 136.65, 139.08, 141.88, 148.84, 150.38, 157.02, 159.31, 165.12. Anal. Calcd. for C20H17N5O: C, 69.96; H, 4.99; N, 20.40. Found: C, 69.83; H, 5.05; N, 20.33.
6-(4-Methoxyphenyl)-4-methyl-6H-benzo[4,5]imidazo[1,2-a]pyrimido[2,1-d][1,3,5]-triazin-2(1H)-one19c.
Yield (81%); DMF (yellow crystals); m.p: 298-300ºC; ir (potassium bromide, cm-1): 3182 (NH), 2970 (CH-aliph.), 1657 (C=O), 1622 (C=N); 1H-NMR (600 MHz, DMSO-d6): δ 2.13 (s, 3H, CH3), 3.70 (s, 3H, OCH3), 5.66 (s, 1H, CH), 5.67 (s, 1H, CH), 6.86-7.38 (m, 8H, Ar-H), 7.87 (s, 1H, NH); Anal. Calcd. for C20H17N5O2: C, 66.84; H, 4.77; N, 19.49. Found: C, 66.67; H, 4.58; N, 19.36.
6-(2-Hydroxyphenyl)-4-methyl-6H-benzo[4,5]imidazo[1,2-a]pyrimido[2,1-d][1,3,5]-triazin-2(1H)-one19d.
Yield (73%); DMF (brown crystals); m.p: 304-306ºC; ir (potassium bromide, cm-1): 3423 (OH), 3210 (NH), 2974 (CH-aliph.), 1657 (C=O), 1610 (C=N); 1H-NMR (600 MHz, DMSO-d6): δ 2.19 (s, 3H, CH3), 5.54 (s, 1H, CH), 6.13 (s, 1H, CH), 7.09-7.39 (m, 8H, Ar-H), 11.52 (s, 1H, NH), 12.43 (s, 1H, OH); Anal. Calcd. for C19H15N5O2: C, 66.08; H, 4.38; N, 20.28. Found: C, 66.15; H, 4.26; N, 20.12.
Synthesis of Benzo[4,5]imidazo[1,2-a]imidazo[2,1-d][1,3,5]triazines20a,b
General procedure: A solution of 3a,b (0.01 mole), 2-hydroxy-1,2-diphenylethan-1-one (0.01 mole) in acetic acid (30 ml) was refluxed for 6 hours. The solid that obtained on cooling was collected by filtration and recrystallized from proper solvent to give compounds 20a,b.
2,3-Diphenyl-3H,5H-benzo[4,5]imidazo[1,2-a]imidazo[2,1-d][1,3,5]triazine20a.
Yield (64%); dioxane (yellow crystals); m.p: 277-278ºC; ir (potassium bromide, cm-1): 3120 (NH), 2971 (CH-aliph.), 1610 (C=N); 1H-NMR (600 MHz, DMSO-d6): δ 6.22 (s, 2H, N(CH2), 7.19-7.57 (m, 14H, Ar-H), 7.97 (s, 1H, NH); Anal. Calcd. for C23H17N5: C, 76.01; H, 4.72; N, 19.27. Found: C, 75.85; H, 4.55; N, 19.15.
5,5-Dimethyl-2,3-diphenyl-3H,5H-benzo[4,5]imidazo[1,2-a]imidazo[2,1-d][1,3,5]-triazine 20b.
Yield (67%); dioxane (yellow crystals); m.p: 281-283ºC; ir (potassium bromide, cm-1): 3142 (NH), 2970 (CH-aliph.), 1630 (C=N); 1H-NMR (600 MHz, DMSO-d6): δ 1.92 (s, 6H, 2CH3), 5.38 (s, 1H, CH), 6.87-7.78 (m, 14H, Ar-H); Anal. Calcd. for C25H21N5: C, 76.70; H, 5.41; N, 17.89. Found: C, 76.61; H, 5.29; N, 17.68.
Analgesic Activity
Mice of either sex (25–30 g) obtained from the animal house at the faculty of pharmacy, Northern Border University were used for this study. The animals were fasted overnight and water ad libitum. The animals were divided into 4 groups, each group containing 10 mice:
Group 1: control (10 ml/kg 2% aqueous gum, oral).
Group 2: positive control (diclofenac sodium 20 mg/kg, oral).
Group 3: Synthesized compound (20 mg/kg, oral).
Group 4: Synthesized compound (40 ml/kg, oral).
Assessment of Analgesic activity
Acetic acid-induced writhing test
The animals were divided into 4 groups with 10 albino mice in each group. Treatment schedule is mentioned in table 1:
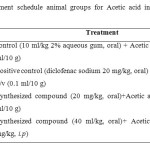 |
Table1: Treatment schedule animal groups for Acetic acid induced wreathing method Click here to View table |
Test samples and 2% aqueous gum administered orally 30 minutes prior to intraperitoneal (i.p) administration of 1.0 % v/v acetic acid (0.1 ml/10 g). Diclofenac sodium administered orally 15 minutes prior to acetic acid. The number of writhes was recorded for 15 minutes commencing just 5 minutes after i.p administration of acetic acid20-22. The percentage protection was calculated as follows:
X1-X2/X1 . 100
X1 = No. of writhing in control group
X2 = No. of writhing in treated group
Acute Toxicity Study:
The acute toxicity study of compound 3c was performed according to OECD/OCDE guidelines No: 423 and a dose of 300 mg/kg body weight was used23. Two groups, each of 3 mice, group-1 is control (animals weighted and administered 10 ml/kg, 2% aqueous gum solution orally) and group-2 administered 300mg/kg of the drug orally (OECD guidelines 423). Animals were observed individually after dosing at 1 hour, 24 hours and 72 hours for behavioral and physiological effects and the observations were recorded23,24.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research, Northern Border University, Kingdom of Saudi Arabia for funding this research work through the research group project No. (435-108-3).
References
- Gaba M., Singh, S. &Mohan C., Eur. J. Med. Chem.2014, 76, 494.
- El-Feky S.A., Thabet H.K. &Ubeid M.T., J. Fluolrine Chem.2014, 161, 87.
- El-Feky S.A., Abd El-Samii Z.K., Osman, N.A., Lashine J., Kamel M.A. &Thabet H.K., Bioorg. Chem.201558, 104.
- Gellis A., Kovacic H., Boufatah N. &Vanelle P., Eur. J. Med. Chem.2008, 43, 1858.
- Curd F.H.S., Graham W.&Rose F.L., J. Chem. Soc.1948, 594.
- Negwar M., Organic-Chemical Drugs and their symptoms. Wiely-VCH VerlagGbmhWeinheim, 2001.
- Nozaki S., Maeda M., Truda H. &Sledze G.W., Breast cancer Res. Treat.2004, 83, 195.
- Dolzhenko A.V., Chui W.K.,Dolzhenko A.V. & Chan L.W., J. Fluorine Chem.2005, 126, 759.
- Atta K.F.M., Farhat O.O.M., Ghobashy S.M., Marei M.G., Molecules, 2011, 16, 10387.
- Abdel-Latif N.A., Sabry N.M., Mohamed A.M. &Abdalla M.M., Monatsh. Chem.2007, 138, 715.
- Amr A.E., Hegab M.I., Ibrahim A.A. &Abdallah M.M., Monatsh. Chem.2003, 134, 1395.
- Tyagi M. &Archana, Orient J Chem. 2015, 31(1), 121.
- Tyagi M., Orient J Chem.2014, 30(2), 713.
- Ibrahim T.S., Rashad A.A., Abdel-Samii Z.K., El-Feky S.A., Abdel-Hamid M.K&Barakat W., Med Chem Res.2012, 4369.
- Elagawany M., Ibrahim M.A., Ahmed H.E.A., El-Etrawy A.S., Ghiaty A., Abdel-Samii Z.K., El-Feky S.A. &Bajorath J., Bioorg. Med. Chem. Lett.2013, 23, 2007.
- Soliman A.M., Mohamed S.K., El-Remaily M.A.A., Abdel-Ghany H.,Eur. J. Med. Chem.2012, 47, 138.
- Nagarajan K., Rao V.R. &Venkateswarlu A., Indian J. Chem.1970, 8, 126.
- Abd El-Latif F.M., Barsy M.A. &Aref A.M., Green Chem.2002, 4, 196.
- Dolzhenko A.V. & Chui W.K., J. Hetercyc. Chem.2006, 43, 95.
- Chang C.W., Chang W.T., Liao J.C., Chiu Y.J., Hsieh M.T., Peng W.H. & Lin Y.C., Evidence-Based Complementary Alternative Medicine, 2012, article ID 135379, 10 pages.
- Zulfiker A.H.M., Rahman M.M., Hossain M.K., Mazumder K.H. &Rana M.S., Biology Medic.2010, 2(2), 42.
- Gupta J.K., Sharma P.K., Dudhe R.,, Chaudhary A. &Verma P.K.,. AnaleleUniversitatii din Bucuresti–Chimie, 2010, 19(2), 9.
- OECD/OCDE 423: OECD Guideline for testing of chemicals. Adopted: 17th December, 2001.
- Bhosle V., Rev. Bras. Farmacogn. Braz. J. Pharmacogn.2013, 23(4), 692.
- Gulyas G., Emri T. &Gyorgydeak Z., Folia Microbiol.2002, 41(1), 29.

This work is licensed under a Creative Commons Attribution 4.0 International License.









