Elimination and Recycling of Imatinib by Ethoxylated Multi-Walled Carbon Nanotubes from aqueous solutions
Maryam Pakzad Masouleh, Shahram Moradi Dehaghi*, Abolghasem Moghimi
Faculty of Chemistry, Tehran North Branch, Islamic Azad University, Tehran, Iran
DOI : http://dx.doi.org/10.13005/ojc/310262
Article Received on :
Article Accepted on :
Article Published : 19 Jun 2015
An adsorbent and carrier based on ethoxylated functionalized multi-walled carbon nanotubes (f-MWCNTs) were prepared with diethylene glycol (2EG) followed by esterification process. Resultant dietylene glycolated MWCNTs (MWCNTs-2EG) used for elimination of Imatinib mesylate (Ima) from water. Maximum Ima adsorption per 3 mg of adsorbent and 9 mg of initial Ima was 8.76 mg. maximum recycling at two pH values 7.4 and 5.3 was determined about 6.15 mg (70%) and 7.42 mg (85%) respectively. Ima elimination and recycling process is greatly enhanced by the creation of functional groups on the MWCNTs-2EG in compare with carboxylated MWCNTs (MWCNTs-COOH).
KEYWORDS:Functionalization; Multi-Walled Carbon Nanotubes (MWCNTs); Ethoxylated; Imatinib (Ima); Elimination; Water; Recycling
Download this article as:| Copy the following to cite this article: Masouleh M. P, Dehaghi S. M, Moghimi A. Elimination and Recycling of Imatinib by Ethoxylated Multi-Walled Carbon Nanotubes from aqueous solutions. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Masouleh M. P, Dehaghi S. M, Moghimi A. Elimination and Recycling of Imatinib by Ethoxylated Multi-Walled Carbon Nanotubes from aqueous solutions. Available from: http://www.orientjchem.org/?p=9320 |
Introduction
Carbon nanotubes as a new emerging nanomaterial have found increasing application in different fields due to their unique chemical and physical properties. Exceptional properties such as high specific area and hollow tube structure made them a suitable candidate for adsorption of different organic and inorganic pollutants [1, 2]. Adsorption is one of the most promising methods among other because of its high efficiency and ease of usage. Carbon nanotubes have been used to adsorption and releasing of molecules, ions and drugs from aqua solutions [3-5]. One of the best ways for improving the adsorption ability of multi walled carbon nanotubes is functionalization. Functionalization of MWCNTs is performed by two different methods consist of physical and chemical. Physical functionalization of MWCNTs involves non-covalent intermolecular interactions but covalent functionalization of MWCNTs is reported for chemical one’s [6-9]. Linlin and et al [10] have been used polyethylene glycolated MWCNTs as an adsorbent for adsorption of oxaliplatin drug.
In this work, we investigated the effect of functionalization of the MWCNTs on elimination rate and recycling of Ima from aqueous solutions in compare with MWCNTs-COOH. Ima has been used for the treatment of chronic myeloid leukemia as a tyrosine kinase inhibitor therapeutically [11]. In order to prepare MWCNTs-2E G, first MWCNTs oxidized to produce MWCNTs-COOH. Then reaction between carboxylate groups of MWCNTs-COOH with oxalyl chloride and after that adding 2EG to this product leads to the formation of MWCNTs-2EG. Then, MWCNTs-COOH and MWCNTs-2EG are dispersed in water and exposure to Ima, separately. Ima adsorption was measured by UV-Vis spectrophotometer. After a while, each product was separated from water by filtration process. After separation of adsorbent from water, the releasing rate of Ima from MWCNTs-2EG measured at pH 7.4 and 5.3. This adsorbent can be used for elimination of organic substances such as Ima from aqueous solutions as useful pollutant and recycled it.
Experimental
Materials
All chemicals are used without further purification. MWCNT (diameter 20-30 nm and length ~30µm) was obtained from CNT Co. Ltd., Incheon, Korea; other reagents and materials were purchased from Merck chemical company.
Instrumentation
Raman spectra were recorded on a Thermo Nicolet model FT-Raman 960. Fourier Transform Infrared Spectra (FT-IR) were recorded using KBr tablets on a Nicolet Nexu 870. FE-SEM images were taken using Hitachi model S-4160. UV-Visible (UV-Vis) absorption spectra were recorded on a Varian Cary 300. Microwave (MW) irradiation was performed using a Milestone Ethos 1. TGA/DSC was recorded on a rheumatic scientific of STA 1500.
Carboxylation of MWCNTs
0.1 g MWCNTs were sonicated in 60 ml of a mixture of H2SO4/HNO3 (3:1 V/V) for 24 h at room temperature. The dark black residue was filtered followed by sonication in H2SO4/H2O2 (4:1 V/V) using a bath sonicator for 30 min. Then, the mixture was centrifuged to separate the functionalized MWCNTs and was washed with deionized water until the pH was neutral. The resulting wet solid sample MWCNTs-COOH was dried under vacuum at 60 °C.
Acylation of MWCNTs.
0.06 g of MWCNTs-COOH in 50 ml Dimethyl Formamide (DMF) was sonicated for 30 min in a bottom flask. Then, 10 ml oxalyl chloride was added to the mixture while stirring under nitrogen gas at -10 °C for 2h. The mixture was stirred at room temperature for 12 h followed by heating at 70 °C for 12 h to remove the excess of oxalyl chloride.
Esterification of MWCNTs
0.06 g of acylated MWCNTs (MWCNTs-COCl) were added to 50 ml DMF. Then, 2EG (9ml) was added to the mixture and sonicated for 45 min. The mixture was microwave (MW) irradiated under atmospheric pressure at 110 °C for 2 h using a 700 w microwave. The black solid product was obtained by filtration (Pore size 0.22 µm).
Ima elimination and recovery
Ima (2 mg and 9 mg) was stirred with 3 mg of MWCNTs-COOH and MWCNTs-2EG in 5 ml aqueous solutions for 6h at room temperature, separately. Then adsorbent were collected by filtration (0.22 µm pore size). The amount of free Ima in water was determined by measuring the absorbance at 257 nm via UV-Vis.
For Ima recycling measurements, the suspensions of adsorbed Ima on functionalized MWCNTs (3 mg) were prepared in water at pH 5.3 and pH 7.4. The suspensions were shaken at 120 rpm at 25 °C and the functionalized MWCNTs were separated by filtration after 5, 24 and 48 h releasing time. The concentration of Ima in the filtrates was quantified using UV-Vis spectroscopy similar to the method reported by Yu-Jen Lu et al [12].
Results and Discussion
SEM
The Field emission scanning electron microscopy (FE-SEM) images of functionalized MWCNTs are observed in Fig. 1. SEM images indicated a new roughness and thickness in functionalized MWCNTs and increasing diameter from 20-30 to 55 nm and about 90 nm for MWCNTs-2EG and Ima adsorbed on MWCNTs-2EG (MWCNTs-2EG-Ima), respectively. Increasing the thickness of functionalized MWCNTs is evident after Ima adsorption.
FT-IR analysis
Fig. 2. 1(a-d) shows the FTIR spectra of MWCNTs, MWCNTs-COOH, MWCNTs-COCl, MWCNTs-2EG. The special FT-IR peaks of functionalized MWCNTs assigned in Fig. 2. 1 (a-d). Fig. 2. 2 (a and b) depicts the FT-IR spectra of Ima (Fig. 2. 2 (a)), MWCNTs-2EG-Ima (Fig. 2. 2 (b)). The functional group analysis of the corresponding FT-IR spectra has been assigned in Fig. 2 (a, b). As it is clear, the characteristic peaks due to the Ima could be clearly observed in the Ima adsorbed on ethoxylated MWCNTs.
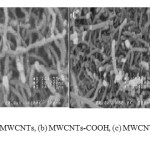 |
Figure1: FE-SEM images of (a) MWCNTs, (b) MWCNTs-COOH, (c) MWCNTs-2EG, (d) MWCNTs-2EG- Ima. Click here to View figure |
 |
Figure2: FT-IR spectra of (1), (a) MWCNTs, (b) MWCNTs-COOH, (c) MWCNTs-COCl, (d) MWCNTs-2EG, FT-IR spectra of (2), (a) Ima, (b) MWCNTs-2EG-Ima. Click here to View figure |
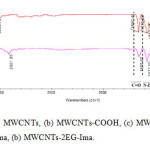 |
Figure2: FT-IR spectra of (1), (a) MWCNTs, (b) MWCNTs-COOH, (c) MWCNTs-COCl, (d) MWCNTs-2EG, FT-IR spectra of (2), (a) Ima, (b) MWCNTs-2EG-Ima. Click here to View figure |
Raman Analysis
The Raman spectra of MWCNTs, MWCNTs-COOH, MWCNTs-COCl, and MWCNTs-2EG and the corresponding data were shown in Fig. 3. The resulting spectra involve the important signals of D-peak and G-peak. The ratio intensity of D-peak to G-peak (ID/IG) in Fig. 3 shows the changing surface structures and reactivity of functionalized MWCNTs. The D-peak was appeared in the 1333-1376 cm-1 region for all structures, The G-band observed in the 1569-1595 cm-1 region [13].
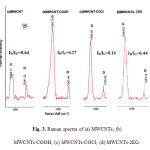 |
Figure3: Raman spectra of (a) MWCNTs, (b) MWCNTs-COOH, (c) MWCNTs-COCl, (d) MWCNTs-2EG. Click here to View figure |
TGA
Fig. 4 shows the TGA and DSC plots of MWCNTs-2EG and the thermal stabilities of MWCNTs-2EG. The solvent vaporization occurred in the temperature range of 27-175 °C. Both decomposition of physical adsorption and ethoxylated MWCNTs lead to weight loss about 10% for MWCNTs-2EG in the temperature range of 175-390 °C. Its results confirmed the physical adsorption on the surface of MWCNTs and esteric bond formation.
 |
Figure4: TGA and DSC plots of MWCNTs-2EG. Click here to View figure |
Ima adsorption by non-covalent bonding and releasing
MWCNTs-2EG was employed for Ima elimination in two initial weights (2 and 9 mg) and the weight of Ima adsorbed on 3mg of adsorbent, were determined 1.62 mg (81%) and 8.76 mg (97%) respectively. The Ima recycling results at two pH values (7.4 and 5.3) in water at 25 °C was shown in Fig. 5. The adsorbed Ima on ethoxylated MWCNTs could be displaced by water molecules and released from the ethoxylated MWCNTs in water [12]. The weight of Ima releasing in water was determined after 5, 24 and 48h using UV-Vis spectroscopy. For MWCNTs-COOH, these process repeated at same method and results discussed in Table 1.
Table1: Efficiency of adsorbents in adsorption, releasing of Ima at 25 °C for 3 mg of MWCNTs-2EG, MWCNTs-COOH.
|
Adsorbents |
mIma0a(mg) |
mIma1b (mg) |
Release, pH=7.4, mg (%) |
Release, pH=5.3, mg (%) |
|||||||||||||||
|
|
5h |
24 h |
48 h |
5 h |
24 h |
48 h |
|||||||||||||
|
MWCNTs-2EG-Ima |
2 |
1.62 |
0.64(43%) |
1.01(62%) |
1.14(70%) |
0.93(57%) |
1.23(76%) |
1.34(83%) |
|||||||||||
|
9 |
8.76 |
2.23(27%) |
5.88(67%) |
6.15(70%) |
3.08(35%) |
6.58(75%) |
7.42(85%) |
||||||||||||
| MWCNTs-COOH-Ima |
2 9 |
0.24 1.44 |
0.09 (37%) 0.32 (22%) |
0.12 (50%) 0.80 (55%) |
0.14 (58%) 1.01 (70%) |
0.11 (45%) 0.40 (27%) |
0.14 (58%) 1.00 (69%) |
0.17 (70%) 1.11 (77%) |
|||||||||||
aWeight of Ima initially solved in water (mg)
bWeight of Ima adsorbed on 3mg of adsorbents (mg)
 |
Figure5: Drug releases profiles of MWCNTs-2EG-Ima (a) and (b); MWCNTs-COOH-Ima (c) and (d) at pH 7.4 and 5.3, at 25 °C per 3mg of adsorbents. (mIma0: Weight of Ima initially solved in water (mg), mIma1: Weight of Ima adsorbed on 3mg of carriers (mg)). Click here to View figure |
The amount of Ima released at pH 5.3 is higher than at pH 7.4 during the recycling measurement. Surface adsorption of ethoxylated MWCNTs is not strong in acidic condition and Ima tend to emigrate from surfaces of ethoxylated MWCNTs and diffuse in water of pH 5.3[12, 14]. Ima was released 7.42 mg (85%) per 3 mg of MWCNTs-2EG and 9 mg of initial Ima. Ima elimination capacity enhanced when the initial weight of Ima increased from 2 to 9 mg (see in Table 1).
Conclusions
In Summary, we have prepared ethoxylated MWCNTS as an Ima adsorbent in aqueous solutions. This esteric functionalizing along with some ethoxylated group creates active sites that can be used as a carrier for elimination of Ima. Maximum elimination capacity of Ima was obtained 8.76 mg per 9 mg of initial Ima and the recovering was found to be 7.42 and 6.15 mg (85% and 70%) per 3 mg of MWCNTs-2EG and 9 mg of initial Ima at 5.3 and 7.4 pH values, respectively. MWCNTs-2EG has unique elimination and releasing properties for Ima. Based on these results, by increasing polarity of the MWCNTs via functionalization, the elimination rate and releasing of Ima in water is increased. Therefore Ima and other toxic compounds can be easily adsorbed and eliminated from water by MWCNTs-2EG.
References
- Salam, M.A.; El-Shishtawy, R.; Obaid, A. J. Ind. Eng. Chem. 2014, 20, 3559–3567.
- Prlainovic´, N. Z.; Bezbradica. D. I.; Knezˇevic´-Jugovic´. Z. D.; Stevanovic´. S. I.; Avramov Ivic´. M. L.; Uskokovic´. P. S.; Mijin. D. Zˇ. J. Ind. Eng. Chem. 2013, 19, 279–285.
- Rashid, H. A and Moghimi, A. Orient. J. chem. 2014, 30, 203-210.
- Fazelirad, H.; Ranjbar, M.; Taher ,M- A and Gh. Sargazi. J. Ind. Eng. Chem. 2015, 21, 889-892.
- Al-Khateeb. L. A.; Obaid. A. Y.; Asiri. N. A.; Salam. M. Abdel. J. Ind. Eng. Chem. 2014, 20, 916-924.
- Shah, M.; Jadhav, N and Kumar, Y. Fullerenes Nanotubes, Carbon Nanostruct, 2009, 17, 528–547.
- Mehra, N. K.; Verma, A. K.; Mishra, P. R and Jain, N. K. Biomaterials, 2014, 35, 4573–4588.
- Shah, M and Agrawal, Y. Fullerenes, Nanotubes, Carbon Nanostruct, 2012, 20, 696–708.
- Zomorod bakhsh , Sh.; Mirza, B.; Yazdizadeh, Sh and Tavahodi, E. Orient. J. chem. 2014, 30 (3), 1379-1383
- Wu, L. L.; Man, Ch.; Wang, H.; Lu, X.; Ma, Q.; Cai, Y and Ma, W. Pharm. Res. 2013, 30, 412–423.
- Roth, O.; Spreux-Varoquaux, O.; Bouchet, S.; Rousselot, P.; Castaigne, S.; Rigaudeau, S.; Raggueneau, V.; Therond, P.; Clin, P.; Chim. Acta. 2010, 411, 140–146.
- Lu, Y. J.; Wei, K.-C.; Mac, C. C. M.; Yang, S.-Y and Chen, J. P. Colloids. Surf B. 2012, 89, 1–9.
- Tan, S. W.; Wang, H. J.; Tu, K. H.; Jiang, H. L.; Wang, Li. Q. Chin. Chem. Lett. 2011, 22, 1123–1126.
- Wong, B. Sh.; Yoong, S. L.; Jagusiak, A.; Panczyk, T.; Ho, H. K.; Ang, W. H and Pastorin, G. Adv. Drug Deliver Rev. 2013, 65, 1964-2015.

This work is licensed under a Creative Commons Attribution 4.0 International License.









