Density functional theory and QM/MM illustration of the behavior of B23N23 nano-cone: EPR & NMR investigation
Samira Bagheri*, Alireza Ziglari
Department of Chemistry, College of science, Central Tehran Branch, Islamic Azad University, Tehran, Iran
DOI : http://dx.doi.org/10.13005/ojc/310228
Article Received on :
Article Accepted on :
Article Published : 06 Jun 2015
In this work, the B23N23 nano-cone has been investigated using the DFT exchange-correlation functional of theory by electron paramagnetic resonance of EPR2 and EPR3 basis sets. The results of ab initio and QM/MM calculations of NMR chemical shift values of σiso, σaniso, Δσ, δ and η parameters of with GIAO and CSGT approximations of B23N23 have been obtained. The relative energies have been compared between EPR2 and EPR3 methods. Also, Atoms charge transfers have considered to full alternation B and N atoms in B23N23 nano-cone. The stability of B23N23 was confirmed in a theoretical work based on a combination of density functional theory and the temperature effect on stability have been studied. The results, obtained of EPR2 and EPR3 levels with both of approximation were excellent in agreement.
KEYWORDS:chemical shift; Boron nitride; nano-cone; ab initio calculations; QM/MM
Download this article as:| Copy the following to cite this article: Bagheri S, Ziglari A. Density functional theory and QM/MM illustration of the behavior of B23N23 nano-cone: EPR & NMR investigation. Orient J Chem 2015;31(2). |
| Copy the following to cite this URL: Bagheri S, Ziglari A. Density functional theory and QM/MM illustration of the behavior of B23N23 nano-cone: EPR & NMR investigation. Available from: http://www.orientjchem.org/?p=9110 |
Introduction
The carbon nanotube (CNT) is a representative nano-material. CNT is a cylindrically shaped carbon material with a nano-metric-level diameter [1-20].
Its structure, which is in the form of a hexagonal mesh, resembles a graphite sheet and it carries a carbon atom located on the vertex of each mesh. The sheet has rolled and its two edges have connected seamlessly [9-15].
Although it is a commonplace material using in pencil leads, its unique structure causes it to present characteristics that had not found with any other materials. CNT can be classified into single-wall CNT, double-wall CNT and multi-wall CNT according to the number of layers of the rolled graphite [16-20].
The type attracting most attention is the single-wall CNT, which has a diameter deserving the name of “nanotube” of 0.4 to 2 nanometers. The length is usually in the order of microns, but single-wall CNT with a length in the order of centimeters has recently released [18-25].
CNT can be classified into single-wall CNT, double-wall CNT and multi-wall CNT according to the number of layers of the rolled graphite. The type attracting most attention is the single-wall CNT, which has a diameter deserving the name of “nanotube” of 0.4 to 2 nanometers [20-26].
The length is usually in the order of microns, but single-wall CNT with a length about centimeters have recently released. The extremities of the CNT have usually closed with lids of the graphite sheet [21-30].
The lids consist of hexagonal crystalline structures (six-membered ring structures) and a total of six pentagonal structures (five-membered ring structures) placed here and there in the hexagonal structure [22-35]. The first report by Iijima was on the multi wall form, coaxial carbon cylinders with a few tens of nanometers in outer diameter [25-40]. Two years later single walled nanotubes were reported [8-45]. SWCNTs have considered as the leading candidate for nano-device applications because of their one-dimensional electronic bond structure, molecular size, and biocompatibility, controllable property of conducting electrical current and reversible response to biological reagents hence SWCNTs make possible bonding to polymers and biological systems such as DNA and carbohydrates [30-50].
boron nitride nanotube (BNNTs) have attracted much interests due to their large gap semi conducting character[41-55].Boron nitride (BN) is a structural existing in cubic (diamond-like), hexagonal (graphite-like), turbo static, and amorphous forms .these compounds have been produced by a variety of methods, such as arc melting[50-59], high temperature chemical reaction[44-60], carbon nanotube templates[50-65], and laser ablating[52-64] The most attention has been focused on the development of new methods for the production of nanotube and inorganic fullerene of other materials. Recently. Lauded and Y. Matsui showed that a non-ablative laser heating method could produce very long BN nanotube assembled in bundles
In addition, theoretical calculations have been described the possible existence of small BN clusters. Jensen and Toftlund [58-70] performed ab initio calculations for B12N12 clusters in different geometries. Based on density functional calculations it has also been proposed that other nanotube could be synthesized. [60-75]
Theoretical studies have been performed for fullerene-like B12N12 clusters,[65-90] in which it has been found that a structure built from squares and hexagons is more stable than those built from pentagons and hexagons. This is because in the second case less stable B-B and N-N bonds are formed.
The most stable B12N12 structure is built from six squares and eight hexagons. [70-99]
In this work, we focused on B23N23 nano-con. Our aim was to obtain the global minimum energy structure. For this structure, we use the hybrid B3LYP exchange-correlation functional within density functional theory. Primary, structure optimization calculated and then Nuclear Magnetic Resonance (NMR) parameters by density Functional Theory (DFT) method calculated on the optimized structure. Isotropic chemical shielding, anisotropic chemical shielding parameters at all of the atoms nuclei are presented in Table 1. And also, Thermodynamic Properties have been considered in Table 2
Table1 .NMR Parameters of B23N23 for 5 atoms of Nitrogen and 5 atoms of Boron with two level of theory
|
|
EPR2 |
|
EPR3 |
|
||||||
|
atom |
|
|
|
|
|
|
|
|
|
|
|
B4 |
161.42 |
85.2 |
-90.79 |
-60.5 |
0.87 |
151.4 |
90.8 |
-92.5 |
-61.7 |
0.9 |
|
N30 |
84.5 |
153.2 |
153.21 |
102.1 |
0.078 |
75.3 |
166.8 |
166.8 |
111.2 |
0.06 |
|
B3 |
30.4 |
333.5 |
333.5 |
222.3 |
0.0589 |
11.9 |
351.63 |
351.6 |
234.4 |
0.03 |
|
N25 |
82.30 |
308.1 |
308.17 |
205.4 |
0.562 |
66.1 |
321.7 |
321.7 |
214.5 |
0.47 |
|
B13 |
82.3 |
308.1 |
308.1 |
205.4 |
0.562 |
66.17 |
321.7 |
321.7 |
214.5 |
0.48 |
|
N39 |
161.4 |
85.1 |
-90.78 |
-60.5 |
0.8 |
151.4 |
90.8 |
-95.5 |
-64.6 |
0.9 |
|
B21 |
76.5 |
49.9 |
-54.7 |
-36.5 |
0.8 |
67.9 |
56.9 |
-63.0 |
-42.0 |
0.8 |
|
N43 |
83.7 |
120.0 |
120.0 |
80.02 |
0.6 |
63.4 |
128.8 |
128.8 |
85.9 |
0.73 |
|
B22 |
-28.8 |
196.5 |
-275.9 |
-183.9 |
0.42 |
-27.8 |
194.3 |
-246.96 |
-164.6 |
0.66 |
|
N45 |
71.9 |
57.8 |
-67.9 |
-45.2 |
0.70 |
63.7 |
61.6 |
-72.9 |
-48.6 |
0.6 |
Table2. Optimized structure parameters of B23N23. For top and down side of Nano-cone
|
bond-angle |
EPR2 |
EPR3 |
|
bond -length |
EPR2 |
EPR3 |
|
N46-B23-N45 |
117.21 |
117.3 |
|
B4-N30 |
1.2 |
1.15 |
|
N29-B3-N25 |
116.98 |
117.0. |
|
B13-N39 |
1.41 |
1.35 |
|
B22-N45-B23 |
117.35 |
117.500 |
|
B3-N35 |
1.39 |
1.36 |
|
B4-N26-B5 |
121.50 |
121.2 |
|
B21-N43 |
1.38 |
1.35 |
DFT calculations of B23N23
B23N23 clusters have been studied using the hybrid B3LYP density functional and (EPR and EPR2) methods. All Density Functional Theory (DFT) quantum calculations were performed using Gaussian 98 program package on structure of boron-nitride nano-cone.
The structure first optimized with Becke3, Lee-Yang-Parr (B3LYP) method and EPRandEPR2 basis sets including QM/MM calculations.
Nuclear Magnetic Resonance (NMR) parameters at all of the nuclei optimized structure calculated by B3LYP method and EPR2 and EPR3 basis sets. The results are listed in table1-4 and Figures1-5.
 |
Figure1: Optimized Structures of B23N23 nano-cone
|
 |
Figure2: contour map of electron density with horizontal projection for B23N23 |
 |
Figure3: Density of state DOS for B23N23 |
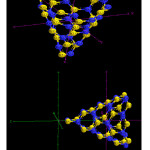 |
Figure4: The B4 and N30 are in the top and B21-N43,B22-N45, B3-N25and B13-N39 are located in the other side |
Table3 .Thermochemistry data for Gibbs energy and enthalpies
|
|
EPR2 |
EPR3 |
|
T(K) |
ΔG( kcal/mol ) |
ΔG( kcal/mol ) |
|
294 |
0 |
0 |
|
296 |
-0.21 |
-0.22 |
|
298 |
-0.42 |
-0.43 |
|
300 |
-0.65 |
-0.67 |
|
302 |
-0.81 |
-0.83 |
|
304 |
-0.95 |
-0.99 |
|
|
|
|
|
|
ΔH( kcal/mol ) |
ΔH( kcal/mol ) |
|
294 |
0 |
0 |
|
296 |
0.09 |
0.09 |
|
298 |
0.21 |
0.22 |
|
300 |
0.29 |
0.29 |
|
302 |
0.39 |
0.39 |
|
304 |
0.49 |
0.49 |
Table4. The diagonal elements are the sum of corresponding row elements Mulliken bond order matrix
|
1 2 3 4 5 1 1.81981537 -0.06694936 0.00121123 0.00004207 -0.06665488 2 -0.06694936 1.91200331 -0.06487913 0.00000003 0.00001287 3 0.00121123 -0.06487913 1.58274924 0.00000000 0.00000337 4 0.00004207 0.00000003 0.00000000 1.95447741 -0.05051190 5 -0.06665488 0.00001287 0.00000337 -0.05051190 1.71119373 6 7 8 9 10 6 -0.04962142 -0.00009760 0.00017808 0.00012890 -0.06624748 7 -0.06510418 -0.06400943 -0.04696122 0.00000090 0.00007086 8 0.00022173 -0.00151502 -0.02522389 0.00000000 0.00000375 9 0.00003142 0.00000088 0.00000001 -0.05619024 -0.06823134 10 -0.00121458 0.00000252 0.00000013 -0.05051190 -0.04415468 11 12 13 14 15 11 1.81981537 -0.06510418 0.00121123 -0.00138446 -0.06100965 12 -0.06510418 1.83299950 -0.04696122 0.00002450 -0.00005600 13 0.00121123 -0.04696122 1.58274924 -0.00000007 0.00000536 14 -0.00138446 0.00002450 -0.00000007 1.78132197 -0.05101726 15 -0.06100965 -0.00005600 0.00000536 -0.05101726 1.78132197 16 17 18 19 20 16 -0.06087757 -0.00224289 0.00006269 -0.00136424 -0.06758153 17 -0.06694936 -0.06400943 -0.06487913 0.00000444 -0.00006233 18 0.00015848 0.00004436 -0.00000061 -0.06758153 -0.00136424 19 -0.00359868 0.00006229 0.00000117 -0.06925424 -0.06925424 20 -0.00000320 0.00001945 0.00000336 0.00003111 -0.00003064 21 22 23 24 25 21 0.00000544 0.00000333 0.00000937 0.00000008 0.00000000 22 0.00000544 -0.00004962 0.00000937 0.00000008 -0.00000003 23 -0.00000095 0.00001945 -0.00000042 0.00000087 0.00000018 24 -0.03414025 0.78789516 -0.00080799 0.00000440 -0.00002473 25 -0.00003615 -0.03664212 -0.00303809 0.00000000 -0.00000000 |
26 27 28 29 30 26 -0.02632326 -0.00002370 -0.00000001 -0.05376705 0.69652041 27 0.76580841 -0.02960282 -0.00003909 -0.00416905 -0.04129322 28 0.77784111 0.77226091 -0.03842573 0.00000845 0.00063358 29 0.00022641 0.72900503 0.83895375 0.00000000 -0.00000010 30 -0.00013587 -0.00000003 -0.00000000 0.59415467 -0.05434021
31 32 33 34 35 31 -0.00430966 -0.00000409 -0.00000001 0.70760248 0.80802830 32 0.76580841 0.00062416 -0.00003909 -0.00416905 0.76218702 33 -0.03414025 -0.00297299 -0.00080799 0.00000440 0.00082141 34 0.00022641 -0.03306726 0.83895375 0.00000000 0.00000194 35 -0.00430966 -0.00003206 -0.00000001 0.70760248 -0.06869552 36 37 38 39 40 36 0.00066316 0.00000236 -0.00000003 -0.02482059 -0.00403887 37 -0.00650259 0.00004714 -0.00000145 0.00118109 -0.03070260 38 -0.00002536 0.00000670 0.00002968 0.00000006 -0.00002011 39 -0.00003615 -0.00002477 -0.00303809 0.00000000 -0.00000021 40 -0.00650259 -0.00117557 -0.00000145 0.00118109 -0.00002055 41 42 43 44 45 41 0.00000637 0.00006459 -0.00000009 -0.00001957 -0.00000225 42 0.00000637 -0.00000187 -0.00000009 -0.00001957 -0.00002661 43 -0.00000004 -0.00000002 -0.00000004 -0.00000000 -0.00000001 44 -0.00002536 -0.00163848 0.00002968 0.00000006 -0.00000014 45 -0.00000004 -0.00000001 -0.00000004 -0.00000000 -0.00000000 46 46 -0.00000001 -0.00000006 0.00000001 -0.00000001 -0.00000000 |
The energy differences have been compared with those obtained within EPR2 and EPR3. The NMR of paramagnetic compounds (compounds possessing unpaired electron) play an important role in different application of chemistry and biochemistry. The stability ofB23N23 was confirmed in a theoretical work based on a density functional calculation. Geometry optimizations and energy calculations on the boron nitrides B23N23 was carried out using the B3LYP method. The major result is that boron nitride cages are more stable than rings if at least two of the six four- member rings are isolated by hexagons.
NMR
The NMR chemical shift d is a parameter that use for recognizing magnetically in equivalent nuclei in a molecule. The use of density functional theory (DFT) to nuclear magnetic resonance (NMR) and electron spin resonance (ESR) spectroscopes is a new and notable subject. Today NMR methods are powerful tools in chemistry and biochemistry because of the NMR chemical shifts. In liquid, or gas, the molecules are freely tumbling so one does comprehend an average chemical shift (isotropic chemical shift). The quantity in quantum mechanics is depended to the NMR chemical shift. The shielding is determined as the mixed second derivative of the energy with consideration to magnetic moment of the nucleus and the strength of the used magnetic field. It is solved through the second-order perturbation theory with the Zee man Hamiltonian.
The first order contribution is called diamagnetic while the second order (which requires knowledge of excited electronic states) is termed paramagnetic. The calculation methods employed are the ab-initio (from first principles) Hartee-Fock or density functional calculations. The first method solves the electronic Schrödinger equation in the absence of any magnetic field. The density functional method was allowed to change with the applied of a magnetic moment and a static external magnetic field. The zero order and first order density matrices are used to obtain the diamagnetic and paramagnetic terms, respectively. The integrity of these calculations depends on the level of theory used, the basis set employed and the structure of molecule. Gaussian basis sets are employed as the basis functions to match the electronic orbital in a molecule.
For use, the ab-initio packages it is necessary that the molecular geometry be defined.
This work presented the results of B and N NMR investigations on the B23N23 nanocone obtained via Density functional theory.
Results
On the basis of the WU Haishun et al studies into (BN)n cages, it is found that among several isomers of these structures the isomers without direct B —B and N —N bonding are more stable. Then, in this work, we have investigated on B23N23 nano-cone.
The B-N bond length at the peak of nano-cone is short to compare to another bonds too in this optimized structure, atomic charges are notable. The charge distribution values of are in agreement with structure coordinate.
Atom charge transfers have considered to full alternation B and N atoms in B23N23 nano-cone. At direct interactions between indicated atoms in EPR3 level have shown that the charges of atoms at ring had the most charge distribution with GIAO and CSGT approximates.
In addition, Atom charge transfers in nitrogen atoms had the same behavior as it performed in tables. This is notable that the atom charge transfer values in top at nano-cone had the close behavior in EPR2 and EPR3 levels.
The agreement between the calculated values Atom charge transfers for EPR2 and EPR3 levels with GIAO and CSGT approximations were remarkably good.
NMR results
The GIAO and CSGT approximations of NMR calculations for Structural study of the B23N23 cluster to obtain nuclei Magnetic Resonance Parameters via Density Functional Theory method were performed at the EPR2 and EPR3 levels and reported in Table1 .the CSGT results have confirm the GIAO results. The agreement between the observed and calculated values of the shielding anisotropies and asymmetries for both EPR2 and EPR3 were remarkably good.
The results of ab initio calculations of NMR chemical shift values of σiso, σaniso, Δσ, δ and η parameters of with GIAO and CSGT approximations of B23N23 have been obtained and results have given by table1.
Calculated isotropic chemical shielding parameters of nitrogen Nuclei have almost similar behaviors and these atoms have maximum values to compare to another atom. In table 1, we have reported the NMR parameters of in EPR2 level and in EPR3 level and also these parameters with CSGT and GIAO approximation are calculated. The same process has been seen in CSGT approximation too. Calculated anisotropic shielding in nitrogen has the same behavior as it performed in table1
It has been shown that the behavior of chemical shift tensor components (isotropic and anisotropic) have depended on the Geometry coordinates of BN nanotube.By given data, we will be able to obtain deeper insight of induced effects of bonding by comparing the calculations.
Thermodynamic Properties
According to the thermochemical calculations at the B3LYP/EPR2 and B3LYP/EPR3 levels of theory, obtaining thermochemical functions such as ΔH, ΔS and ΔG.
Also, in this work the changes of Gibbs free energy and Enthalpy via temperature have considered and have shown that with increasing of temperature ,the system has been stabilized according to the (Eq.1) as follows .ΔG= ΔH –TΔS : Eq.1.Gibbs equation
Conclusions
The hybrid B3LYP density functional and (EPR2 and EPR3) methods has been used to characterize the geometry of a B23N23 nanocone. Optimized structures, relative stability, nuclear dipole moment and NMR parameters of system including total atomic charges, shielding isotropies, shielding anisotropies chemical shift and asymmetry of considered system have been compared and results have been in good agreement with the experimental data.
References
- Massimo, Fusaro .:Quantum Matter,2014, 3, 481-487
- Micheal Arockiaraj , Rev. Theor. Sci. 2014, 2, 261-273
- Martin Bohlén .; Kim Bolton ,Quantum Matter, 2014, 3, 339-343
- Monajjemi, M.; Baei, M.T.; Mollaamin, F. Russian Journal of Inorganic Chemistry. 2008, 53 (9), 1430-1437
- Monajjemi, M.; Rajaeian, E.; Mollaamin, F.; Naderi, F.; Saki, S. Physics and Chemistry of Liquids. 2008, 46 (3), 299-306
- Monajjemi, M.; Seyed Hosseini, M. Journal of Computational and Theoretical Nanoscience .2013 ,10 (10), 2473-2477
- Yahyaei ,H.; Monajjemi, M. Fullerenes, Nanotubes, and Carbon Nanostructures.2014, 22(4), 346–361
- Monajjemi, M .; Jafari Azan, M.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures.2013, 21(6), 503–515
- Bhupesh, Bishnoi .; Bahniman ,Ghosh .Quantum Matter.2014, 3, 469-475
- Sule, Celasun , Rev. Theor. Sci.2013, 1, 319-343
- Akshaykumar, Salimath .; Bahniman, Ghosh, Quantum Matter, 2014, 3, 72-77
- Nafisi, S.; Monajemi, M.; Ebrahimi, S. Journal of Molecular Structure. 2004,705 (1-3) 35-39
- Monajjemi , M.; Baheri ,H.; Mollaamin ,F. Journal of Structural Chemistry.2011 52(1), 54-59
- Monajjemi, M.; Seyed Hosseini, M.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures. 2013, 21, 381–393
- Monajjemi, M.; Boggs, J.E. J. Phys. Chem. A, 2013, 117, 1670 −1684
- Davide Fiscaletti and Amrit Sorli ,Quantum Matter,2014 3, 200-214
- Monajjemi , M.; Honaparvar , B.; Khalili Hadad ,B.; Ilkhani ,AR.; Mollaamin, F. Afr. J. Pharm. Pharmacol .2010, 4 (8), 521-529
- Monajjemi, M. Chemical Physics. 2013, 425, 29-45
- Bjürn Piglosiewicz, Jan Vogelsang.;Slawa Schmidt.; Doo Jae Park.; Petra Groß, .; Christoph Lienau ,Quantum Matter,2014 ,3, 297-306
- A. M. Ilyin , Quantum Matter 2013,2, 205-208
- Fazaeli ,R.; Monajjemi ,M.; Ataherian ,F.; Zare, K. Journal of Molecular Structure: THEOCHEM.2002, 581 (1), 51-58
- Monajjemi, M.; Mollaamin, F, J Clust Sci, 22(2011)673.
- Medhat Ibrahim and Hanan Elhaes ,Rev. Theor. Sci. 2013, 1, 368-376
- Anurag Srivastava.; Nileshi Saraf.; A. K. Nagawat , Quantum Matter,2013, 2, 401-407
- IIJIMA Sumio.; YUDASAKA Masako.; NIHEY Fumiyuki.; NEC TECHNICAL JOURNAL,2007, 2,1,
- S. Iijima, Nature,1991, 354, 56.
- S. Iijima and T. Ichihasi, Nature,1993,363, 603
- D. S. Bethune.;C. H. Kiang.; M. S. deVries.; G. Gorman, R. Savoy.; J. Vazques.; R. Beyers, Nature ,1993, 363, 605
- T. Ramanathan.; F. T. Fisher.; R. S. Ruoff and L. C. Brinson, Chem mater,2005 17, 1290
- Monajjemi, M .; Aghaie , H.; Naderi , F. Biochemistry (Moscow).2007, 72 (6), 652-657
- Monajjemi, M. Journal of Molecular Modeling , 2014, 20, 2507
- Davide Fiscaletti,Rev. Theor. Sci.2013 1, 103-144
- Monajjemi , M.; Chahkandi ,B.; Zare,K.; Amiri, A. Biochemistry (Moscow),2005 70 (3), 366-376
- J.-Y. Guo, C.-X. Xu, F.-Y. Sheng, Z.-L. Jin, Z.-L. Shi, J. Dai, and Z.-H. Li Quantum Matter,2013 2, 181-186
- Monajjemi, M .; Afsharnezhad ,S.; Jaafari , M.R.; Abdolahi ,T.; Nikosade ,A.; Monajemi ,H.; Russian Journal of physical chemistry A, 2007, 2,1956-1963
- Monajjemi, M.; Khaleghian, M, Journal of Cluster Science. 2011, 22 ( 4 ), 673-692
- Mollaamin , F.; Monajjemi , M , Journal of Computational and Theoretical Nanoscience. 2012, 9 (4) 597-601
- Monajjemi, M. Struct. Chem, 2012, 23 551.
- Mollaamin, F.; Gharibe, S.; Monajjemi, M. Int. J. Phy. Sci , 2011,6, 1496-1500
- Monajjemi, M .; Faham, R.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures , 2012 20, 163–169
- Monajjemi, M.; Khaleghian, M.; Tadayonpour, N.; Mollaamin, F. International Journal of Nanoscience, 2010, 9 (05), 517-529
- Mollaamin , F .; Najafi ,F.; Khaleghian, M.; Khalili Hadad, B.; Monajjemi ,M. Fullerenes, Nanotubes, and Carbon Nanostructures,2011 19, 653–67
- Monajjemi, M.; Chegini , H. ; Mollaamin , F. ; Farahani ,P, Fullerenes, Nanotubes, and Carbon Nanostructures.2011,19, 469–482
- Monajjemi, M.; Yamola ,H.; Mollaamin,F. Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22, 595–603
- Monajjemi, M.; Heshmata, M.; Haeri, HH , Biochemistry (Moscow),2006, 71 (1), S113-S122
- Xinjun Wang.; Yi Xie and Qixun Guo , CHEM. COMMUN. 2003, 2688–2689
- A. Rubio.; J.L. Corkill.; M.L. Cohen.; Phys. Rev. B ,1994, 49, 5081
- X. Blasé.; A. Rubio.; S.G. Louie.; M.L. Cohen.; Europhys. Lett, 1994.28, 335.
- N.G. Chopra.; J. Luyken.; K. Cherry.; V.H. Crespi.; M.L. Cohen,S.G. Louie.; A. Zettl, Science.1995 , 269, 966
- N.G. Chopra.;A. Zettl.;Solid State Commun.1998 ,105, 297
- J. Cumings.; A. Zettl, Solid State Commun.2004, 129, 661
- R. Ma.; Y. Bando.; H. Zhu.; T. Sato, C. Xu, D. Wu, J. Am.Chem. Soc.2002, 124, 7672,
- L. Wirtz, A. Rubio, R.A. de la Concha, A. Loiseau, Phys. Rev.B 68 ,045425,(2003).
- P.W. Fowler, K.M. Rogers, G. Seifert, M. Terrones, and H. Terrones, Chem. Phys. Lett. 1999, 299, 359
- Monajjemi, M .; Falahati, M.; Mollaamin, F.; Ionics, 2013 , 19, 155–164
- Monajjemi , M.; Heshmat ,M.; Aghaei , H.; Ahmadi , R.; Zare,K. Bulletin of the Chemical Society of Ethiopia, 2007, 21 (1)
- Monajjemi , M.; Lee, V.S. ; Khaleghian, M.; B. Honarparvar, B.; F. Mollaamin, F, J. Phys.Chem. C. 2010, 114 (2010) 15315
- A. Rubio, J.L. Corkill, M.L. Cohen, Phys. Rev. B.1994, 49,5081
- X. Blase, A. Rubio, S.G. Louie, M.L. Cohen, Europhys. Lett. 1994, 28 335.
- N.G. Chopra, J. Luyken, K. Cherry, V.H. Crespi, M.L. Cohen,S.G. Louie, A. Zettl, Science ,1995, 269, 966.
- O.R. Lourie, C.R. Jones, B.M. Bartlett, P.C. Gibbons, R.S. Ruoff,W.E. Buhro, Chem. Mater.2000, 12 ,1808;
- R. Ma, Y. Bando, T.Sato, Chem. Phys. Lett.2001, 337 ,61.
- W. Han, Y. Bando.; K. Kurashima.; T. Sato, Appl. Phys. Lett.1998, 73, 3085;
- D. Golberg.; Y. Bando.; W. Han.; K. Kurashima.;T. Sato, Chem. Phys. Lett.1999. 308 337 D. Golberg, Y. Bando, K.Kurashima, T. Sato, Chem. Phys. Lett.2000 323) 185.
- (a) D. Golberg.; Y. Bando.; M. Eremets.; K. Takemura.; K. Kurashima.;H.Yusa, Appl. Phys. Lett.1996 ,69, 2045
- D.P. Yu, X.S. Sun, C.S. Lee, I. Bello, S.T. Lee, H.D. Gu, K.M. Leung, G.W. Zhou, Z.F. Dong.; Z. Zhang.; Appl. Phys. Lett.1998, 72 , 1966.
- F.Jensen.; H.Toftlund, Chem. Phys. Lett.1993, 201,89 , 94
- Monajjemi, M .; Sobhanmanesh, A .; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures,2013, 21 47–63
- Monajjemi ,M.; Karachi ,N.; Mollaamin, F. Fullerenes, Nanotubes, and Carbon Nanostructures, ,2014, 22: 643–662
- Monajjemi, M.; Mahdavian, L.; Mollaamin, F.: Bull .Chem.Soc.Ethiop ,2008, 22(2),1-10.
- V. Tozzini.; F. Buda.; A. Fasolino.; physical review letters ,2000,21,85
- G. Seifert.; P. W. Fowler.; Mitchell, D.; Porezag, D.; Frauenheim, Th. Chem. Phys. Lett. 1997, 268, 252
- Mollaamin ,F.; Baei, MT.; Monajjemi, M.; Zhiani , R.; Honarparvar , B.; Russian Journal of Physical Chemistry A, Focus on Chemistry,2008, 82 (13), 2354-2361
- Monajjemi, M.; Ghiasi, R. ;Ketabi, S. Journal of Chemical Research.2004, 1: 11-18
- Mahdavian,L.; Monajjemi, M.; Mangkorntong ,N.Fullerenes, Nanotubes and Carbon Nanostructures,2009, 17 (5), 484-495
- Jon M. Matxain.; Jesus M. Ugalde.; M. D. Towler.; and R. J. Needs.; J. Phys. Chem. A 2003, 107, 10004-10010
- WU Haishun.;XU Xiaohong,; JIAO Haijun,
- ZHANG Fuqiang .; JIA Jianfeng. Chinese Science Bulletin. 2003, 48, 11 1102 1107
- Monajjemi, M .; Ketabi ,S.; Amiri, A. Russian Journal of Physical Chemistry , 2006, 80 (1), S55-S62
- M. Monajjemi .; Robert Wayne Jr, J.E. Boggs, Chemical. Physics. 433 (2014) 1-11
- Jon M. Matxain.; Jesus M. Ugalde.; M. D. Towler.; and R. J. Needs.; J. Phys. Chem. A 2003, 107, 10004-10010
- WU Haishun.; XU iaohong.; JIAO Haijun, ZHANG Fuqiang .; JIA Jianfeng Chinese Science Bulletin 2003, 48 (11), 1102 1107
- Monajjemi , M.; Honarparvar, B.; Monajemi, H.;. Journal of the Mexican Chemical Society, 2006, 50 (4), 143-148
- Monajjemi ,M.; Mollaamin ,F. Journal of Computational and Theoretical Nanoscience,2012 ,9 (12) 2208-2214
- Monajjemi, M.; Mahdavian, L.; Mollaamin, F.; Honarparvar, B. Fullerenes, Nanotubes and Carbon Nanostructures, 2010, 18, 45–55
- Friedrich, B.; J.D. Weinstein.; R. Decarvalho .; J.M. Doyle.;. Trap. J. Chem. Phys. 1999, 110,2376-2383.
- Ramsay, N.E.; Magnetic Shilding of Nuclei. J. Phys. Rev.1950, 78, 699-703.
- Frischend, M.J.; J.B. Foresman, 1995. Gaussian 94 user’ reference (Gaussian, Inc., Pittsburgh).
- Ghalandari, B.; Monajjemi, M.; Mollaamin, F.; Journal of Computational and Theoretical Nanoscience, 2011 8, 1212–1219
- Monajjemi , M.; Khosravi , M.; Honarparvar, B.; Mollaamin, F.; International Journal of Quantum Chemistry, 2011, 111, 2771–2777
- Monajjemi, M.; Rajaeian, E.; Mollaamin, F. Physics and Chemistry of Liquids,2008, 46 299.
- Tahan, A .; Monajjemi, M. Acta Biotheor, 2011, 59, 291–312
- Monajjemi, M.; Farahani, N.; Mollaamin, F. Physics and Chemistry of Liquids, 2012, 50(2) 161–172
- Monajjemi, M.; Razavian, M.H.; Mollaamin,F.; Naderi,F.; Honarparvar,B.; Russian Journal of Physical Chemistry A , 2008 , 82 (13), 2277-2285
- Mollaamin , F.; Varmaghani , Z.; Monajjemi , M, Physics and Chemistry of Liquids. 2011, 49 318
- Monajjemi, M.; Honarparvar, B.; H. Haeri, H.; Heshmat, M.; Russian Journal of Physical Chemistry C, 2008, 80(1),S40-S44.
- Cheeseman, J.R.; M.J. Frisch.; F.J. Devlin .;P.J.Stephens, Chemical Physics Letters, 1996,252 (3-4), 211-220.
- Pisani, C.;S. Casassa .; P. Ugliengo, Chemical Physics Letter. 1996, 253 (3-4) 201-208.
- Dresselhaus, M.; Dresselhaus, G.; Eklund, P. C., Science of Fullerenes and Carbon Nanotubes, San Diego: Academic Press, 1996, 109, 175.

This work is licensed under a Creative Commons Attribution 4.0 International License.









