The interaction of single walled carbon nanotube (SWCNT) with phospholipids membrane: in point view of solvent effect
Akbar Elsagh1*, Hamidreza Jalilian2, Ali. R. Ilkhani3
1Department of Chemistry, North Tehran Branch, Islamic Azad University, Tehran, Iran
2Department of Chemistry, Gorgan Branch, Islamic Azad University, Gorgan, Iran
3Department of Chemistry, Yazad Branch, Islamic Azad University, Yazad, Iran
DOI : http://dx.doi.org/10.13005/ojc/310124
Article Received on :
Article Accepted on :
Article Published : 19 Mar 2015
In this research, we have studied the structural properties of phospholipids, surrounding single-walled carbon nanotube (SWCNT, by using ab-inition and molecular dynamics simulation. Carbon nanotubes (SWCNTs) are very common in medical research and are being highly studied in the fields of biosensing methods for disease treatment and efficient drug delivery and health monitoring. The transportation of SWCNT through the cell membrane widely investigated because of many advantages. Because of the differences among force fields, the energy of a molecule calculated using two different force fields will not be the same. In this study difference in force field illustrated by comparing the energy of calculated by using force fields, MM+, Amber and OPLS. The quantum Mechanics (QM) calculations were carried out with the GAUSSIAN 09 program based on density functional theory (DFT) at B1LYP/6-31G* level. In our recent study the electronic structure of open-end of SWCNT and transportation of SWCNT through the phospholipids in skin cell membrane have been discussed for both vacuum and solvent media.
KEYWORDS:The interaction; biosensing methods; phospholipids
Download this article as:| Copy the following to cite this article: Elsagh A, Jalilian H, Ilkhani A. R. The interaction of single walled carbon nanotube (SWCNT) with phospholipids membrane: in point view of solvent effect. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Elsagh A, Jalilian H, Ilkhani A. R. The interaction of single walled carbon nanotube (SWCNT) with phospholipids membrane: in point view of solvent effect. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=7846 |
Introduction
Nature provides us with a very large number of channels or nano-pores embedded in cell membranes. The function of channels is to allow selectivity and specificity for a Variety of molecular species transport across the cell membrane. These channels,included : a) ligand-gated channels)voltage-gated channels, c)Second messenger gated channels, d)mechanic sensitive channels)Gap junctions: porins not gated [1-7].
The discovery of carbon nanotubes (CNT) in 1991 heralded the era of nanoscience and nanotechnology [8-11].
Nanotubes of carbon and other materials, due to their electronic, optical and mechanical properties find applications in several fields [12-18].
The space available inside the nanotube enables it to match phospholipids outside. This makes the carbon nanotube an ideal medium for storing high energy materials [19-21].
Carbon nanotubes (CNTs) since their discovery [22] have been the focus of scientific research due to their outstanding chemical, mechanical and electrical properties. Single walled carbon nanotubes (SWCNTs) are of particular interest because of their size and superior electrical properties [23].SWCNTs have been used to realize many molecular scale electronic devices [24 -29].
Membranes are asymmetric structures. The choline-containing phospholipids are located mainly in the outer molecular layer .This asymmetric distribution is maintained by an ATP-dependent protein which specifically Trans-locates phosphatidyl-ethanolamine (and phosphatidyl-serine) to the inside of the plasma membrane [30-33].
All major lipids in membranes contain both hydrophobic and hydrophilic regions and are therefore termed amphipathic. Dipalmitoylphosphatidylcholine (DPPC) and dimyristoylphosphatidylcholine (DMPC) are taken as phospholipids with an equal polar heads and with the difference in the length of hydrocarbon chains [34,35].These two molecules have saturated fatty acid tail groups [36-39].
On a molecular level it has been of interest to explore to what extent PC head groups differ with respect to molecular conformation, lateral interactions, and dipole arrangements and how these features affect the properties and topology of the membrane surface [40-44].
There are two types of CNTs: single-walled nanotubes (SWCNTs) and multi-walled nanotubes (MWCNTs) [45]; that they have three conformation: armchair (n,n), zigzag (n,0) and chiral (n,m) these conformations have individual properties [46-50].
SWCNTs have been considered as the leading candidate for nanodevice applications because of their one-dimensional electronic bond structure, molecular size, and bio compatibility, controllable property of conducting electrical current and reversible response to biological reagents hence SWCNTs make possible bonding to polymers and biological systems such as DNA and carbohydrates [51-57].
Computational Methods
All calculations have done by ab initio at the Hartree-Fock (HF) level of theory Gaussian 98 package[58].Four basis sets have used, namely the sto-3G ,3-21G,6-31G and 6-31G*. The geometry of DPPC, DMPC have full optimized at the RHF/6-31G*, 6-31G, 3-21G and STO-3G levels of the theory in the gas phase [59-61].
The most important dihedral angle of these molecules (DPPC and DMPC) is chosen and
The energy, dipole moment, and atomic charges of 15 important atoms have scanned within 180 degrees rotation. In this manner after the optimization of total molecules, important dihedral angle of these molecules has rotated 15 degrees at every time [62-64].
The effects of the solvent polarization are described in terms of proper QM operators to be added to the Hamiltonian of the isolated system then , the salvation calculations have performed using Onsager method at HF/6-31 G*. For Onsager model, it does require values of volume (a0) of the molecule and the dielectric (ε) of solvent. The volume of DPPC and DMPC molecules was obtained using the “volume” keyword. The Onsager-SCRF was that it permitted one to directly exploit almost all of the computational facilities of the Gaussian packages .For this reason, and for its very limited computational cost , it is still in use by people not requiring an accurate description of salvation effects but just a guess or a qualitative correction to the values obtained for the isolated molecule. Users must be aware of the limitations of the approach,of the unphysical deformation of the solute charge distribution it may induce, and of other shortcomings specific of the approach, such as the lack of salvation for solutes with zero permanent dipole [65-66].
In the Onsager method, the solute molecule is placed in a spherical cavity of radius a0 surrounded by a continuum with constant dielectric properties [67]. A dipole in the molecule will induce a dipole in the medium, and the electric field applied by the solvent dipole will in turn in interact with the molecular dipole leading to net stabilization. The molecular geometrics have obtained via HF/6-31 G* level optimization in the gas phase and have rotated and then any one separately have been placed in the solvents [67, 68].
Result and Discussion
Dipalmitoylphosphatidylcholine (DPPC) and dimyristoylphosphatidylcholine (DMPC) molecules have chosen as starting structures for gas phase (Fig1).
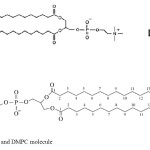 |
Figure1: DPPC and DMPC molecule Click here to View figure |
The DPPC and DMPC zwitter-ionic are found to be unstable in the gas phase when have optimized at HF/3-21G, 6-31G and 6-31G* level.The obtained result from optimization and stabilization parameters are shown in table1.
Table 1. Conformational energy of DPPC and DMPC obtained by geometry optimization for different basis set.
|
Basis set |
E/Kcal.mol-1 |
E/Kcal.mol-1
|
|
DPPC |
DMPC |
|
|
Sto-3G |
-1583811.25 |
-148406.13 |
|
3-21G |
-1594584.734 |
-149458.5 |
|
6-31G |
-1602683.01 |
-1505418.6 |
|
4-31g |
-1604567.05 |
-1505341.9 |
|
6-31G* |
-1603386.341 |
-1505422.7 |
|
6-311++G** |
-1605567036 |
-160552.9 |
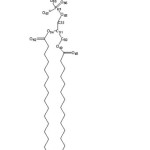 |
Figure2: of DPPC Click here to View figure |
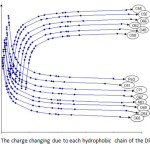 |
Figure3: The charge changing due to each hydrophobic chain of the DPPC Click here to View figure |
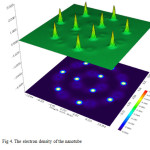 |
Figure4: The electron density of the nanotube Click here to View figure |
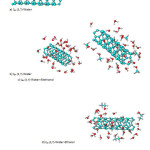 |
Figure5: The Nano tubes in various solvent of water and methanol Click here to View figure |
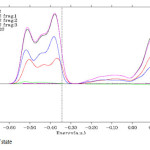 |
Figure6: Density of state Click here to View figure |
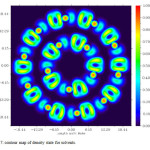 |
Figure7: contour map of density state for solvents. Click here to View figure |
References
- L.K.Kaczmarek, T.M.perney, curr.opin.cell.Biol.3 (1991)663.
- D.A.Doyle,J.moraiscabral, Science 280 (1998)69.
- Kratschmer W, Lamb LD, Fostiropoulos K, Huffman DR.Solid C60-a new form of carbon. Nature 347, (1990) 354.
- MajidMonajjemi, Robert Wayne, JrandJames E. Boggs, Chemical Physics ,433 (2014),1-11.
- T. Ardalan , P. Ardalan& M. Monajjemi,Fullerenes, Nanotubes, and Carbon Nanostructures, 22: 687–708, 2014
- M. Monajjemi, S. Ketabi, M. HashemianZadeh, and A. Amiri, Biochemistry (Moscow), 2006, 1,Suppl 1, S1-S8.
- M. Monajjemi, S. Ketabi, A. Amiri ,Russian Journal of physical chemistry , 2006(1),55-62.
- S. Iijima, Nature 354 (1991) 56.
- M. Monajjemi, Struct Chem., 23 (2012) 551-580.
- M. Monajjemi , N. Karachi & F. Mollaamin, Fullerenes, Nanotubes, and Carbon Nanostructures, 22: 643–662, 2014.
- M. Monajjemi, J.E Boggs, J. Phys. Chem A. 117 (2013) 1670.
- M. Monajjemi, L. Mahdavian, F. Mollaamin and M. Khaleghian ,Interaction of Na, Mg, Al, Si with Carbon Nanotube (CNT):NMR and IR Study Russian Journal of Inorganic Chemistry, 2009, 54(9), 1465–1473.
- F. Mollaamina, Z. Varmaghani,and M. Monajjemi.Dielectric effect on thermodynamic properties in vinblastine by DFT/Onsager modeling, Physics and Chemistry of Liquids, ,2011,49(3):318-336.
- Monajjemi M, Lee V S, Khaleghian M, Honarparvar B, Mollaamin F(2010). Theoretical Description of Electromagnetic Nonbonded Interactions of Radical, Cationic,and Anionic NH2 BHNBHNH 2 Inside of the B 18 N18Nanoring. J. Phys. Chem. C. 114: 15315–15330.
- M. Monajjemi, L. Mahdavian, F.Mollaamin. Characterization of nanocrystallinecylicon germanium film and nanotube in adsoption gas by montecarlo and langevin dynamic simulation Bull. Chem. Soc. Ethiop, 2008, 22(2), 1-10.
- M. Monajjemi, M. SeyedHosseini& F. Mollaamin, Fullerenes, Nanotubes, and Carbon Nanostructures, 21: 381–393, 2013
- F. Mollaamin , M. Monajjemi& J. Mehrzad , Fullerenes, Nanotubes, and Carbon Nanostructures, 22: 738–751, 2014.
- M. Monajjemi; H. Chegini; F. Mollaamin; P. Farahani , Fullerenes, Nanotubes, and Carbon Nanostructures, 19: 469–482, 2011
- H.A. Rachid, A. Hu, V. Timoshevskii, Y. Song, L.S. Lussier, Phys. Rev. Lett. 100, (2008) 204.
- Majid Monajjemi, FatemehMollaamin , TaherehKarimkeshteh, J. Mex. Chem. Soc. 2005, 49(4), 336-340
- V.S.Lee,P.Nimmanpipug,F.Mollaamin,N.Kungwan,S.Thanasanvorakun, and M. Monajjemi ,Russian Journal of Physical Chemistry A, 2009, 83, 13, 2288–2296.
- Iijima S. Helical microtubules of graphitic carbon. Nature 354, (1991) 56.
- Odom TW, Huang JL, Kim P, Lieber CM. Atomic structure and electronic properties of single-walled carbon nanotubes. Nature 39, (1998) 62.
- Postma HWC, Teepen T, Yao Z, Grifoni M, Dekker C. Carbon nanotube single-electron transistors at room temperature. Science 293, (2001) 76.
- M. Monajjemi, A. Sobhanmanesh, & F. Mollaamin, Fullerenes, Nanotubes, and Carbon Nanostructures, 21: 47–63, 2013.
- F. Mollaamin, K. Shahani pour, K. Shahani pour, A. R. Ilkhani, Z. Sheckari, and M.Monajjemi, Russian Chemical Bulletin, International Edition, 61 (12) , 2012.
- M. Monajjemi, F. Mollaamin, J ClustSci, 22(2011)673 .
- H. Yahyaei , M. Monajjemi , H. Aghaie, and K. Zare , Journal of Computational and Theoretical Nanoscience,10(10), 2332–2341, 2013.
- M. Monajjemi& M. Falahati& F. Mollaamin, Ionics (2013) 19:155–164
- A.Zachowski, E.Favre, S.Cribier, P.Hevre´, P. F.Devaux, J.Biochemistry 1986, 25, 2585-2590.
- Monajjemi,M. Honarparvar, B. H. Haeri, H. Heshmat ,M. (2006) Russian Journal of Physical Chemistry C.,80(1):S40-S44.
- A. Tahan and M. Monajjemi, ActaBiotheor (2011) 59:291–312
- M. Monajjemi, R. Sayyadi, G. Ghasemi, Kh.Lalateh, A. Nouria, F. Naderi,MAIN GROUP METAL CHEMISTRYVOLUME ,28(5): 2005 247-263
- M. A. Kiselev,P. Lesieur, A. M. Kisselev and M. Ollivon, Ice Formation in Model Biological Membranes in the Presence of Cryoprotectors ,Nuclear Instruments & Methods in Physics Research ., 2000,A 448,225-260.
- H. yun, Y.w. Choi,N. J. Kim.and D. Sohn., Physicochemical properties of Phosphatidylcholine(PC)Monolayers with Different Alkyl Chains,at the Air/Water Interface., Bull. Korean hem. Soc.2003, 24, 3 ,337.
- J. Landin and I. Pascher ,Effect of a Polar Environment on the Conformation of Phospholipid Head Groups Analyzed with the Onsager Continuum Solvation Model. J. Phys. Chem. A 1997, 101, 2996-3004
- M. Monajjemi, E. Rajaeian, F. Mollaamin, F. Naderi, and S. Saki., Phys. Chem. Liq. 46, 3299 (2008).
- M.Monajjemi,M.Heshmat,H.Aghaei,R.Ahmadi,K.Zare,Bulletin of the Chemical Society of Ethiopia 21 (1), pp. 111-116, 2007
- Monajjemi, M., Aghaie, H.,Naderi, F , Biochemistry (Moscow) 72, 2007(6),799-804.
- A.J. Robinson, W.G. Richards, P.J. Thomas ,M.M.Hann,Head group and chain behavior in biological membranes: a molecular dynamics computersimulation. Biophys J. 1994 ,67,6,2345–2354
- MONAJJEMI M, Zare K, Gharib F,JOURNAL OF CHEMICAL AND ENGINEERING DATA 40 (2): 419-422(1995).
- M. Monajjemi, F. Naderi, F. Mollaamin, and M. Khaleghian,J. Mex. Chem. Soc. 2012, 56(2), 207-211
- Monajjemi M, Azizi Z, Ghavami M,RUSSIAN JOURNAL OF INORGANIC CHEMISTRY 48 (10): 1551-1559 ,2003
- M. Monajjemi, F. Naderi, H. Aghaie, M. Yari, D.N. Mansoor, S. Afsharnezhad, F. Mollaamin, Research Journal of Chemistry and Environment 11 (2), (2007),56-62.
- M. Foley, Cheap Tubes, 2005.
- B. Gojman, H. Hsin, J. Liang, N. Nezhdanova and J. Saini, 2004.
- F. Mollaamin , F. Najafi , M. Khaleghian , B. KhaliliHadad& M Monajjemi , Fullerenes, Nanotubes, and Carbon Nanostructures, 19(7): 653–667, 2011
- H. Yahyaei& M. Monajjemi,Fullerenes, Nanotubes, and Carbon Nanostructures, 22(4): 346–361, 2014
- M. Monajjemi, and M. Ahmadianarog, Journal of Computational and Theoretical Nanoscience 11(6), 1465-1471, 2014
- B. Ghalandari, M. Monajjemi, and F. Mollaamin, Journal of Computational and Theoretical Nanoscience,8, 1212–1219, 2011
- T. Ramanathan, F. T. Fisher, R. S. Ruoff and L. C. Brinson, Chem mater, 17, 1290, (2005).
- M. Monajjemi, M.H.Razavian, F.Mollaamin, F.Naderi, B.Honarparvar, Russian Journal of Physical Chemistry A 82 (13), 2277-2285, 2008.
- M. Monajjemi, J. Najafpour& F. Mollaamin., Fullerenes, Nanotubes, and Carbon Nanostructures 21(3): 213–232(2013).
- F. Mollaamin and M. Monajjemi ,Journal of Computational and Theoretical Nanoscience , 9 (4) 597-601 ,2012.
- M. Monajjemi , R. Faham& F. Mollaamin, Fullerenes, Nanotubes, and Carbon Nanostructures, 20: 163–169, 2012
- M. Monajjemi, and F. Mollaamin, Journal of Computational and Theoretical Nanoscience ,9 (12) 2208-2214 ,2012.
- M. Monajjemi, H. Baheri, and F. Mollaamin, Journal of Structural Chemistry. 52(1), 54-59, 2011.
- M.J. Frisch, G.W.Trucks, H.B.Schlegel, G.E. Scuseria, M.A. Robb, J.R Cheeseman, V.G.Zakrzewski,J.A. Montgomery, Jr., R.E. Stratmann, J.C.Burant,`S. Dapprich, J.M. Millam, A.D. Daniels, K.N. Kudin,M.C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi , R.Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J.Ochterski, G. A. Petersson, P.Y. Ayala, Q. Cui, K.Morokuma, D.K. Malick,A. D. Rabuck, K. Raghavachari, J.B. Raghavachari, J. Cioslowski, J. V. Ortiz, A.G. Baboul,B.B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi,R. Gomperts, R.L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, C. Gonzalez, M.Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W.Wong, J. L. Andres, C. Gonzalez, M. Head-Gordon, E. S.Replogle, J. A. Pople. Gaussian, Inc., Pittsburgh PA, 1998.
- Majid Monajjemi, Chemical Physics,425 (2013),29-45.
- M. Monajjemi , M. Heshmat ,H. H. Haeri,Biochemistry (Moscow), 2006,(71), S113-S122.
- Monajjemi M, Ghiasi R, Abedi A,Russian Journal of Inorganic Chemistry,50(3),pp. 382-388 (2005).
- M.Monajjemi,S.Afsharnezhad,M.R.Jaafari,T.Abdolahi,A.Nikosade and H.Monajemi, Russian Journal of physical chemistry A, 2007(2),1956-1963.
- Monajjemi, M. Ghiasi, R. Ketabi, S.(2004). Journal of Chemical Research.1: 11-18 .81. Wadt, W. R and Hay, P. J. (1985) J. Chem. Phys, 82: 284.
- Majid Monajjemi, Chemical Physics,425 (2013),29-45.
- Monajjemi M, Chahkandi B, Zare K,Amiri A.,Biokhimiya 70(3),447-458, 2005
- Monajjemi, M., Afsharnezhad, S., Jaafari, M. R., Mirdamadi, S., Mollaamin, F. and Monajemi, H. (2008) .Chemistry 17(1): 55-69.
- M.Sundaralingam, Molecular structures and conformations of the phospholipiods and Sphingomyelins. Ann. NY Acad. Sci. 1972, 195,324-355.
- Monajjemi M, Chahkandi B,JOURNAL OF MOLECULAR STRUCTURE-THEOCHEM 714 (1): 43-60 ,2005

This work is licensed under a Creative Commons Attribution 4.0 International License.









