Stable oxygen and hydrogen isotope fractionation factors for the Goethite (hematite)-water system
Khawar Sultan1,2
1Department of Hydrology, Universität Bayreuth, Universitaetsstrasse 30, 95440 Bayreuth, Germny.
2School of Marine and Environmental Sciences, University Malaysia Terengganu, 21030 Kuala Terengganu, Malaysia.
DOI : http://dx.doi.org/10.13005/ojc/310107
Article Received on :
Article Accepted on :
Article Published : 21 Mar 2015
This paper reports experimental results on the low-temperature (<100 ºC) δ18O and δ 2H fractionation of goethite (hematite)-water in a closed system. Both goethite (α-FeOOH) and hematite (α-Fe2O3) exhibited closer fractionation factor values but the αHematite-Water value is slightly higher (~0.9932) than the αGoethite-Water (~0.9924) for the 18O isotope. The average fractionation factor (1000 ln2α; at 70 ºC) value for 2H in the goethite-water is determined to be -115.78 which is more negative than the 1000ln18α values for 18O. The isotopic change from initial waters to the final waters in which these minerals were synthesized, was observed to be larger for the δ2H (average~2.02‰) than the δ18O (average~0.55‰). Variations in the fractionation factors of goethite and hematite reported in various studies is probably related to the procedures such as drying, washing, type of reactants, pH, and extraction and measurement of 18O and 2H isotopes and, therefore, invite further research for the understanding of α-T relation. Formation temperatures of goethite (~70 ºC) and hematite (~90 ºC) seem to have less impact in altering mineral-water fractionation as compared to the formation water.
KEYWORDS:Goethite; Hematite; Hydrogen and Oxygen isotope; Fractionation factor; Temperature
Download this article as:| Copy the following to cite this article: Sultan K. Stable oxygen and hydrogen isotope fractionation factors for the Goethite (hematite)-water system. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Sultan K. Stable oxygen and hydrogen isotope fractionation factors for the Goethite (hematite)-water system. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=7969 |
Introduction
The high reactivity of iron with oxygen results in various Fe-(hydr)oxides in surface and near surface environments. Among these commonly occurring Fe-minerals, the low solubility of the goethite (α-FeOOH) and hematite (α-Fe2O3), under oxidizing conditions preserve the isotopic information on ancient environments [1, 2]. The isotopic ratios (e.g., 18O/16O, D/H) of these crystallized FeIII-minerals reflect the original temperature of formation and the isotopic signature of the formation water [3, 4]. Isotopic ratios of goethite and hematite may closely mimic the isotopic fractionation exhibited in many natural environments and, therefore, are required to understand paleotempratures and isotopic composition of the waters present at the time Fe-(hydr)oxide formation. Being authigenic minerals in both continental and oceanic settings, goethite and hematite are important repository of knowledge of geologic environments. A well defined fractionation-temperature relation is vital if isotopic composition of goethite and hematite are to provide quantitative information.
This work investigates into the isotopic signature of both oxygen and hydrogen in synthetic goethite and hematite. It includes an attempt to determine fractionation factor at a specific temperature, and isotopic composition of waters present at the time of mineral formation.
Material and Methods
Hematite (α-Fe2O3) and goethite (α-FeOOH) were prepared by aging 2-line ferrihydrite from the alkaline FeIII systems following the methods of Schwertmann and Cornell [5]. Goethite was synthesized from 100 mL of 1 M Fe(NO3)3.9 H2O in 2 L polyethylene bottle. About 180 mL 5 M KOH was added and the suspension was diluted to 2L. The suspension was held at 70 ºC (pH~ 13) for 60 hours. Reactants were preheated to the designated temperature before mixing. Hematite was synthesized by dissolving 40 g of Fe(NO3)3.9H2O in 500 mL distilled water and adding 300 mL of 1M KOH. To this was added 50 mL 1M NaHCO3 and the suspension was kept in a closed polyethylene flask at 90 ºC for 48 hours (pH~ 8-8.5). Both hematite and goethite were synthesized in two types of waters such as Milli Q (type-I) and ultra pure (type –II) with different isotopic values. The end products were centrifuged and washed to remove electrolyte (OH, NO3, CO3, Na and K) repeatedly. Separation of phases (precipitate) of the synthesized minerals was performed using an ultra speed centrifuge. Samples were then dried under vacuum in a freeze drier and grounded. The presence of NO3was tested qualitatively with diphenylamine.
Oxygen and hydrogen isotopes of initial and final waters (type-I and type-II; n= 12) in which goethite and hematite were synthesized were also measured along with the solid end products (n =4; GI, GII, HI and HII). The isotope analysis of samples was conducted at the BayCEER laboratory, University of Bayreuth, Germany. The samples were ground (<100µm), dried and the TC-IRMS coupling was used for the simultaneous determination of oxygen (δ18O) and hydrogen (δ2H) isotope abundances. Each sample was weighed into the silver capsule, tightly closed and introduced into the pyrolysis oven. There sample was thermally converted to CO and H2 for the H and O isotope analysis, respectively, under the oxygen free conditions. The gases thus produced were purified in a chemical trap and separated by gas chromatography subsequently. The relative abundances of the H and O isotopes were analyzed by the isotope ratio mass spectrometry (IRMS). The isotope ratios are presented in the delta notation as given below:
δX = [Rsample/Rstandard – 1] X 1000‰
Where δX is the δ value of the heavy isotope X (δ18O or δ2H) and R is 18O/16O or 2H/H .The standard is V-SMOW (Vienna Standard Mean Ocean Water) [6]. Overall analytical precision is ±0.2‰ for δ18O and ±0.3‰ for δ2H measurements. Specific surface areas of goethite and hematite samples were determined by gas adsorption using Brunauver-Emmett-Teller (BET) specific surface analysis instrument (Micromeritics, USA). Each dried sample was treated in a mixed-gas flow (N2 31% and He 70%; flow rate 70ml/min) at 150 ºC for 15 min and cooled to liquid nitrogen temperature (-196 ºC). The mineralogy and purity of the samples were investigated by X-ray powder differaction (XRD), Fourier Transform Infra Red (FT-IR) spectroscopy and SEM.
Results
Product description
This method produced 8.21 g goethite and 7.43 g hematite with the average surface areas of 23.5 and 30.9 m2/g, respectively. X-ray diffractograms and IR spectra of goethite and hematite samples are shown in Figure 1 and 2. Both goethite and hematite samples showed sharp identifiable X-ray diffraction (XRD) peaks. All peaks in the IR spectra correspond to hematite and goethite and are indistinguishable from each other revealing uniformity of the experimental conditions. The goethite consists of relatively large acicular crystals (300 ~ 600 nm long; 60 ~ 100 nm wide: 15 ~ 20 nm thick.). Hematite crystals were fairly uniform in size (30 ~ 60 nm) and diamond shaped. SEM images of four samples are shown in Figure 3.
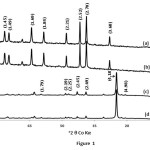 |
Figure1: X-ray diffractograms of pure hematite ( (a) in type-1 and (b) in type-II water ) and goethite ((c) in type-1 and (d) in type-II water) synthesised from FIII salt solutions. The “d” spacings are in parentheses (in Å). Click here to View figure |
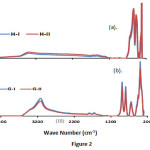 |
Figure2: Infrared spectra of pure hematite (a) and goethite (b) samples. Click here to View figure |
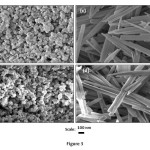 |
Figure3: SEM images of pure hematite ( (a) in type-1 and (b) in type-II water ) and goethite ((c) in type-1 and (d) in type-II water). Hematite crystals are small platelets with some diamond shaped. Acicular goethite crystals (c & d) cut perpendicular to the needle axis. Click here to View figure |
Goethite
Oxygen and hydrogen isotope data for pure synthetic minerals and water samples are listed in Table 1. The δ18OGoethite values varied from -16.75 to -14.99‰ and the corresponding waters in which goethites were synthesized varied between -9.114 (final water ) and -6.84‰ (initial water). The δ2HGoethite varied from –167.10 to -160.14‰, while the corresponding waters ranged from -65.96 to -55.20‰.
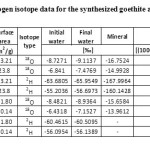 |
Table1: Oxygen and hydrogen isotope data for the synthesized goethite and hematite. Click here to View table |
The average isotopic separation, defined here as ΔGoethite–Water = δGoethite -δWater, between goethite and water was -7.58 ‰ for 18O and -102.61‰ for 2H. The average fractionation factor (αGoethite–Water) is measured to be 0.9924 for the 18O isotope and 0.8907 for the 2H isotope. Yapp [7] reported 18αGoethite-Water value of 1.0022‰ at 62 ºC which is higher than the value measured in this study. The average fractionation factor (2αGoethite–Water) for the 2H isotope is measured to be 0.8907. Yapp [8] reported the fractionation factor (2αGoethite–Water) value of 0.905 at 62 ºC which is much higher than the value measured in this study. At present there are no other published experimental values of 2αGoethite–Water available for comparison.
Hematite
The δ18OHematite values varied between -15.66 and -13.96‰ (range~ 1.7‰) and the corresponding waters in which hematite was synthesized varied from -8.94 to -6.43‰ (Table 1). No δ2H values were measured for hematite. The δ2H values for initial and final water in which hematites were synthesized did not show any significant change (≤ 0.048‰) revealing no active role of hydrogen during the mineral formation.
The average isotopic separation (ΔHematite–Water) between hematite and water is -6.77‰ for 18O isotope. The average fractionation factor (αHematite–Water) for the 18O isotope is measured to be 0.9932‰. Zheng [9] reported a much lower 18αHematite–Water value of 0.9889 ‰ at 90 ºC and Yapp [7] reported a much higher value of 0.9998 ‰ at 92 ºC.
Discussion
The δ18O values showed a positive correlation with δ2H in both water and iron oxide samples. The δ18O and δ2H values of all goethite and hematite samples shifted in apparent response to the isotopic composition of the initial water in which the mineral was in contact (Fig. 4). The magnitude of the change in δ18O is slightly lower for the goethite (0.39 and 0.64‰) than the hematite (0.45 and 0.72‰) samples. The magnitude of change in δ2H is much larger than δ18O (Fig. 5). A slight change in temperature results in a larger variation in hydrogen than the oxygen due to the large difference in masses. Both δ18O and δ2Hvalues of final waters were lower than the initial waters which indicated the incorporation of heavy O- and H- isotopes into the solid products, goethite and hematite. The δ2H values of initial and final waters did not change due to no active role playing in the hematite (Fe2O3) crystal growth.
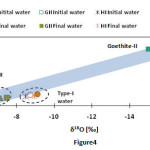 |
Figure4: Plot of δ18O vs. δ2H of water and iron oxide samples. Click here to View figure |
![Fig. 5: Isotopic difference [ δFinal water - δInitial water ] in 18O and 2H values between the initial and final water in which goethite and hematite were synthesized.](http://www.orientjchem.org/wp-content/uploads/2015/03/Vol31_No1_Stab_KHAWA_Fig5-150x150.jpg) |
Figure5: Isotopic difference [ δFinal water – δInitial water ] in 18O and 2H values between the initial and final water in which goethite and hematite were synthesized. Click here to View figure |
The goethite and hematite exhibited closer fractionation factor values with water. The higher isotopic values of the type-II water showed relatively higher fractionation factors (α> 0.9915‰) as compared to the type-I water. The αHematite–Water value is slightly higher (~0.001; Fig. 6(a)) than the αGoethite–Water for the 18O isotope. The hematite was synthesized at higher temperature (~90 ºC) than the goethite (~70 ºC). Two minerals also differ in the synthesis pathway after the initial formation of ferrihydrite (Fe5HO8.H2O approx.). The hematite is formed by the direct solid state transformation from the ferrihydrite by internal reorganization. Therefore, the 18O isotope of hematite is solely related to the 18O isotope of the initial water from which precursor ferrihydrite formed. The goethite is formed by the dissolution of ferrihydrite and subsequent precipitation as goethite [10]. In the goethite crystal growth process the isotopic composition of the initial ferrihydrite may be lost. Also, goethite crystals took longer (~60 h) to form than the hematite (~48 h) and, therefore, mineral-water isotopic equilibrium may have been approached. The enrichment factor (εFe-oxide-Water) decreased systematically with the increase of fractionation factor reflecting isotopic signature of the initial water in which the minerals were synthesized. Hematite samples showed both higher fractionation and enrichment factors as compared to the goethite samples (Fig. 6(a)). The δ18OMineral showed a positive correlation with δ18OWater . In terms of mineral-water relationship both goethite and hematite showed a similar trend by plotting along the line (Fig. 6(b)).
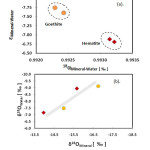 |
Figure6: Plots of the fractionation factor against the enrichment factor of mineral-water system (a) and 18O of mineral against water (b). Click here to View figure |
The fractionation factor (1000ln18α) and temperature relation revealed slightly lower value of the goethite-water as compared to the hematite-water (Fig. 7). The two ferric (hydr)oxides were synthesized at different temperatures which may explain differences in 1000ln18α values. Various studies presented 1000ln18α values for goethite-water and hematite-water which differ from the values determined in this study. A comparison is shown in Figure 7. The mineral-water oxygen isotope fractionation factors calculated from the α-T relations given by various researchers ranged from -8 to 2 for goethite ( at 70 ºC) and -11.0 to 1.5 for hematite ( at 90 ºC). Yapp [7, 8] presented the α-T relation for both goethite and hematite-water system and he concluded that O-isotope fractionation factors for these two minerals are identical. Zheng [9, 11] calculated the 1000ln18α values for goethite-water which are significantly different from the hematite-water (Fig. 7). The average fractionation factor (1000ln2α) value for hydrogen in the goethite-water is determined to be -115.78‰ which is more negative than the 1000ln18α values for oxygen.
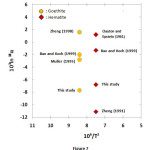 |
Figure7: Plots of 103ln 18α vs. 106/T2 for goethite-water (~70 ºC) and hematite-water (~90 ºC) fractionation factors determined by synthesis experiments and calculations. Click here to View figure |
The most likely reasons for a wide range in fractionation factors at the same temperature are due to the difference in procedures followed to obtain δ18O and δ 2H data. Which include drying, washing, type of reactants, pH, and extraction and measurement of 18O and 2H isotopes in a sample. Moderate to low temperatures synthesis experiments may never reach isotopic equilibrium due to the extremely low rates of mineral-water exchange [4] so α-T relation at lower temperature may not represent the true equilibrium. Formation temperatures of goethite (~70 ºC) and hematite (~90 ºC) seem to have less impact in altering mineral-water fractionation as compared to the formation water.
Conclusions
Goethite-water fractionation factor values for 18O and 2H isotopes are measured to be 0.9924 and 0.8907, respectively. Hematite-water fractionation factor value for the 18O is measured to be 0.9932. These values differ from the values reported in the literature probably due to the differences in the synthesis conditions. The isotopic change is much larger for the δ2H than the δ18O in waters in which minerals were synthesized. Formation temperatures of goethite and hematite seem to have less impact in altering mineral-water fractionation as compared to the formation water.
Acknowledgments
The author would like to thank the Alexander von Humboldt Foundation (AvH), Germany, for the financial support in carrying out the research work. Thanks also to Prof. Dr. Stefen Peiffer and Prof. Dr. Gerhard Gebauer for their support regarding the isotope analysis.
References
- Clayton, R.N.; Epstein, S. Journal of Geology. 1961, 69, 447-452.
- Yapp, C. Annual Review of Earth and Planetary Sciences. 2001, 29, 165-199.
- Yapp, C. J. Geochimica et Cosmochimica Acta. 1998, 62, 2409-2420.
- Bao, H.; Koch, P. Geochemica et Cosmochimica Acta. 1999, 63, 599-613.
- Schwertmann, U.; Cornell, R.M. Iron Oxides in the Laboratory: Preparation and Characterization, Second edition, Wiley-VCH, Germany, 2000.
- Gonfiantini, R. Nature, 1978, 271, 534-536.
- Yapp, C. J. Chemical Geology, 1990, 85, 329-335.
- Yapp, C. J. Geochimica et Cosmochimica Acta, 1987, 51, 355-364.
- Zheng, Y. F. Geochimica et Cosmochimica Acta, 1991, 55, 2299-2307.
- Schwertmann, U.; Murad, E. Clays and Clay Minerals, 1983, 31, 277-284.
- Zheng, Y.F. Physics and Chemistry of Minerals, 1998, 25, 213-221.

This work is licensed under a Creative Commons Attribution 4.0 International License.









