Fused Heterocyclic Nitrogen Systems Containing Phosphorus Atom
Dina A. Bakhotmah* ,Reda Mohammadi. Abdel-Rahman
Chemistry Department, Faculty of Science, King Abdul-Aziz University Jeddah
DOI : http://dx.doi.org/10.13005/ojc/310101
Article Received on :
Article Accepted on :
Article Published : 10 Feb 2015
Synthesis of fused heterocyclic nitrogen systems containing phosphorus atom via [1+4] Cyclocondensation, [3+2] cycloaddition, [3+2] cycloaddition and 1,5-electrocyclization reactions are reviewed. Also, the modified synthesis methodology applicable to the fused phospha-five and six-membered rings.
KEYWORDS:Synthetic; Fused; Phosphaheteocyclization; phosphorus
Download this article as:| Copy the following to cite this article: Bakhotmah D. A, Abdel-Rahman R. M. Fused Heterocyclic Nitrogen Systems Containing Phosphorus Atom. Orient J Chem 2015;31(1). |
| Copy the following to cite this URL: Bakhotmah D. A, Abdel-Rahman R. M. Fused Heterocyclic Nitrogen Systems Containing Phosphorus Atom. Orient J Chem 2015;31(1). Available from: http://www.orientjchem.org/?p=7113 |
Introduction
Organophosphorus compounds Research has steadily flourished[1] because these compounds have been reported to possess anti-TMV activity2, herbicides3,4, insecticides5,6, molluscidal7, and some of here useful as strong basic proazaphosphatrans8, complexes9,10 also as superior catalyst of the protective silyation of wide variety of sterically hindered and deactivated alcohols11.
Fused and isolated heterocyclic nitrogen systems have been testified as anticancer12,13 and plant protection14 activities. In addition, phosphorus containing heterocyclic compounds exposed interesting biologically active15, 16. Thus, in the present review an attempt has been made to highlight the synthesis and properties of fused heterocyclic systems containing phosphorus atom.
Distribution
The Synthetic strategy for new organophosphorus heterocyclic systems was analogous to the Krohnke`s synthesis17 via [4+1] cyclo condensation, [3+2] cycloaddition18 and 1,5-electrocyclization19. Bansal et al20,21 and abdel-Rahman22 reported a brief reviews about the synthesis of indolizines and phosphaindolizines.
Synthesis via [4+1] cyclocondensation reactions are most important. Thus, synthesis of 2-phosphoindolizines (3) was deduced from condensation of 1,2-dialkylpyridinium bromide (1) with PCl3 in presence of Et3N via intermediate 2 [Scheme 1]23, 24.
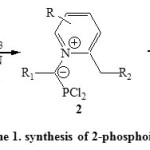 |
Scheme1: synthesis of 2-phosphoindolizines Click here to View scheme |
If R2 is methyl group, that reaction proceeds through the intermediate 5 to yield 1-dichlorophosphino-2-phosphoindolizine (6) [Scheme 2]25.
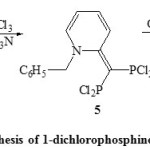 |
Scheme2: synthesis of 1-dichlorophosphino-2-phosphoindolizine Click here to View Scheme |
Similarly, 1,3-azaphospholo[5,1-b]thiazolines (7) and 1,3-azaphos-pholo[5,1-b]benzothiazoles (8) were synthetic26.
On the other hand, 1-phosphaindolizines (10) was obtained from condensation of 1,3-oxazolo[3,2-a]pyridinium salt (9) with P(SiMe3)3 via exchange oxygen by phosphorus27.
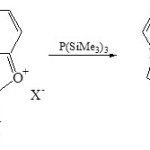 |
Scheme 2A Click here to View scheme |
A related condensation of 1-alkyl-2-aminopyridinium salt (11) with PCl3 in presence of Et3N led to the formation of 1-aza-2-phosphindolizines (13) via the intermediate 12. [Scheme 3]28.
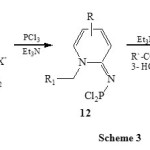 |
Scheme 3 Click here to View scheme |
A large number of 3-aza-2-phosphaindolizines (15) were prepared29 from condensation of 2-alkyl-1-aminopyridinium iodides (14) with PCl3 in presence of Et3N.
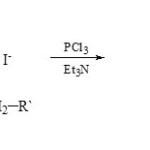 |
Scheme 3A Click here to View scheme |
Also, 3-aza-phosphaindolizines (17) have been synthetic via O/P exchange of 1,3,4-oxadiazolo[3,2-a]pyridinium salts (16) with P(SiMe3)330.
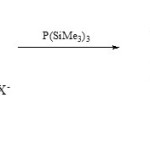 |
Scheme 3B Click here to View scheme |
It is interesting that phospha-fused heterobicyclic as 1,3-diaza-2-phosphaindolizines (19) were obtained from cyclocondensation of 1,2-diaminopyridinium iodides (18) with P(NMe3)3 in boiling dry benzene31.
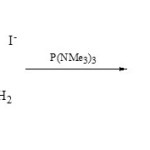 |
Scheme 3C Click here to View scheme |
Karaghiosoff et al32 reported the using of chloromethyl dichloro-phosphine (ClCH2PCl2) as equivalent reagent yielding two-heterocyclic fused via C – P moiety by cyclocondensation with 2-substituted azoles and azines through [3+2]cyclocondensation reactions33.
Thus, 2-aminopyridine (20) condenses with (ClCH2PCl2) in presence of Et3N to produce 1,4,2-diazaphospholo[4,5-a]pyridine (21)34.
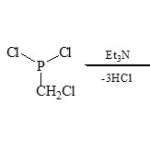 |
Scheme 3D Click here to View scheme |
On the other hand, [3+2] cycloaddition reactions were used tert-butylphospha acetylene 1,3-dipolarophile to condense with pyridinium (22) to give 2-phosphaindolizine (23) by boiling in dry toluene18.
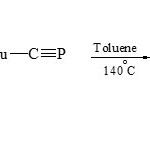 |
Scheme 3E Click here to View scheme |
Azomethine ylides and azomethine imines incorporate that structure of 1,3-dipolar moiety bonded directly to an olefinic or acetylenic bond possesses 1,5-dipolar structure and have been utilized for the synthesis of five-membered heterocycles19, 35
Thus, disproportionation of N-pyridinium dichlorophosphonium-methylides (24) would generate the bis-(N-pyridiniumylidyl) phosphonium chloride (25) which on disproportionation via 1,5-electrocyclization by loss the pyridinium hydrochloride to yield 2-phosphaindolizine (26) [Scheme 4]36.
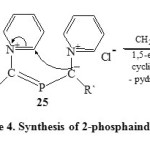 |
Scheme4: Synthesis of 2-phosphaindolizine Click here to View scheme |
Abdel-Rahman22, reported the synthesis of new phosphaheterobi-cyclic system containing 1,2,4-triazine moiety via heterocyclizationa, this in cloud a novel synthesis of fluorine bearing 5-phospha-1,2,4-triazin-3-thiones and 5-phospha-1,2,4-triazepin-3-thione.
Thus, a facile synthesis of fused phosphaheterobi-cyclic nitrogen system containing 1,2,4-triazine moiety was describe earlier. The treatment N-substituted thiosemicarbazides37 (27) with diethyl benzoyl phosphate and/or diphenyl(2,4,6-trimethyl benzoyl) phosphine oxide in boiling dry toluene or THF38 afforded 4,5,6-trisubstitued-5-phospha-1,2,4-triazin-3(2H)thiones (28 & 29) respectively. Refluxing both the compounds 28 and 29 with trifluoroacetic anhydride yielded39 the full fluorinated phospha-1,2,4-triazinethiones 30 and 31 respectively [Scheme 5]22.
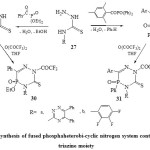 |
Scheme5: synthesis of fused phosphaheterobi-cyclic nitrogen system containing 1,2,4-triazine moiety
|
correspondingly, cyclocondensation of N 4-substituted thiosemicarbazide (27) with acetonyltriphenylphosphonium chloride in boiling THF-DMF40 led to the direct formation of hexahydro-4,7-disubstituted-5-triphenyl-5-phospha-1,2,4-triazepin-3-(2H)thione (32)22.
Presence of both NH and CH2 groups in the compound 32 was deduced from acylation and or condensation with trifluorobenzaldehyde40 to give the final products fluorine-bearing 1,2,4-triazepin-3-thione derivative (34) [Scheme 6]22.
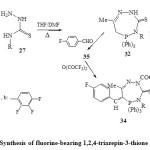 |
Scheme6: Synthesis of fluorine-bearing 1,2,4-triazepin-3-thione derivative Click here to View scheme |
Regioselective iminophosphoran-mediated annelation of a 1,3,4-thiadiazole ring into a 1,2,4-triazine ring was studied by Molina et al42. These studies based on aza-Wittig type reaction of iminophosphoane (37) followed by addition isothiocyanate-formed mesomeric or Zwitter ionic character 35 and 36. Treatment of compound 36 with primary aromatic amines afforded the Betaine 37 which under treatment with ethanol in the presence of tetrafluoro boric acid by stirring for 7 hours, resulted the 1,3-thiadiazetidines 38 via carbodiimide under goes [2+2] cycloaddition reaction [Scheme 7]42.
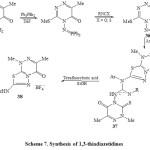 |
Scheme7: Synthesis of 1,3-thiadiazetidines Click here to View scheme |
From survey and various publications, it showed that a lethal work on the area of phospha-1,2,4-triazines41-43. The first study between phosphorus and 5,6-diphenyl-1,2,4-triazin-3(2H) one (39) and some phosphorus reagents such as TPP, P(OR)3 and (CH2)2O2PZ (Z = Cl, OMe, N(Me)2) were isolated phospha-heterocyclic system 40 obtained43.
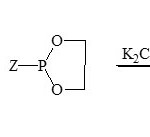 |
Scheme 7A Click here to View scheme |
The pesticides are a group of chemicals intended for perveting/destroying production, processing storage transportation and distribution of food. Thus, thiophosphate pesticides are the most effective insecticides and are used to control a wide variety of insect’s pest as Parathion6 (41). Also the insecticide as Dimethaoate5 (42).
 |
Scheme 7B Click here to View scheme |
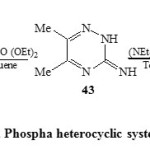
Several studies 12,13,44 found that fused heterobicyclic nitrogen systems have a wide spectrum of medicinal and biological activities. Thus, treatment22 of 3-amino-5,6-dimethyl-1,2,4-triazine (43) with triphenylphosphine dibromide in toluene38 afforded compound 44, while refluxing of 43 with (EtO)3P=O in dry toluene45 yielded compound 45 [Scheme 8]22.
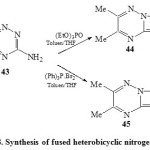 |
Scheme8: Synthesis of fused heterobicyclic nitrogen systems Click here to View scheme |
In addition, condensation of 3-amino-triazine 43 with aromatic aldehyde and (p-ClC6H4)3P and/or acetophenone/ArPCl2 in presence of dry toluene40,46-TEA led to the direct formation of phospha heterocyclic systems 46 and 47 respectively [Scheme 9]23. Formation of compound 46 may be takes place as shown in Scheme 1047.
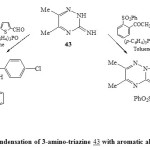 |
Scheme9: Condensation of 3-amino-triazine 43 with aromatic aldehyde Click here to View scheme |
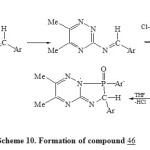 |
Scheme10: Formation of compound 46 Click here to View scheme |
In addition, the reaction of 3-amino-5,6-dimethyl-1,2,4-triazine (43) with diethyl benzyl phosphonate and/or (NEt2)3P+ Br– in dry toluene produced the phospha heterocyclic systems 48 and 49 respectively [Scheme 11]23.
 |
Scheme11: Phospha heterocyclic systems Click here to View scheme |
On the other hand, 3-amino-6-methyl-5-styryl-1,2,4-triazine (50) react with CS2 in alcoholic KOH to give (6-methyl-5-styryl-1,2,4-triazin-3-yl)dithiocarbamic acid (51), which work as a building block for fused phospha-heterobicyclic systems 52-57 as bi-six-membered rings22. Thus, compound 51 on treatment with phosphorus reagents such as (Ph)3PBr2, P(NR2)3 and (MeO)3P=O in refluxing toluene-TEA furnished 1,2,4-triazino[2,3-c][1,2,5,2]thiadiapho-sphorin-6-thiones (52-54) respectively [Scheme 12]22.
![Scheme 12. Synthesis of 1,2,4-triazino[2,3-c][1,2,5,2]thiadiapho-sphorin-6-thiones](http://www.orientjchem.org/wp-content/uploads/2015/02/Vol31_No1_FUSE_Dina_sch12-150x150.jpg) |
Scheme12: Synthesis of 1,2,4-triazino[2,3-c][1,2,5,2]thiadiapho-sphorin-6-thiones Click here to View scheme |
The behavior of marcapto group in compound 51 towards halogenated phosphorus reagents to produce compounds 55-57 is similar to its reaction with various alkylating agents and or ketonic agents. It is worthy of mention that nucleophilic attack on SH is more than NH group by chlorinated phosphorus reagents48. Thus, refluxing compound 51 with CNCH2P(O)(OEt)2, PhCH2P(O)(OEt)2 and Br– P+(NEt2)3 in THF-DMF furnished the target compounds 55-57 [Scheme 13]22.
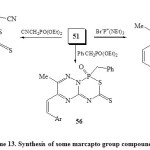 |
Scheme13: Synthesis of some marcapto group compound Click here to View scheme |
The tautomeric structures and proton mobility in position 3 and 2 of 1,2,4-triazines considered to be important in heterocyclization process with halogenated phosphorus reagents. 22 Also, the reactivity structure relationship showed that phospha-1,2,4→triazine similar as Atranes which have unexpected properties49.
Ibrahim et al50, reported the synthesis of novel 1,2-thiaphos-pholo[4,5- e][1,2,4]triazines (60) by treatment of 1,2,4-triazin-6-one (58) and or 1,2,4-triazin-6-thione (59) with different percentage of Lawesson`s reagent (LR)51 [Scheme 14].
![Scheme 14. synthesis of novel 1,2-thiaphos-pholo[4,5- e][1,2,4]triazines](http://www.orientjchem.org/wp-content/uploads/2015/02/Vol31_No1_FUSE_Dina_sch14-150x150.jpg) |
Scheme14: synthesis of novel 1,2-thiaphos-pholo[4,5- e][1,2,4]triazines Click here to View scheme |
The molecular modifications of 1,2,4-triazine rings by introducing organophosphorus functionalities might be expected to exhibit the potential activities depending on the position of the phosphoryl group to 1,2,4-triazine ring, Abdel-Rahman et al6, studied synthesis of phosphorus containing fused and isolated heterobicyclic nitrogen systems via reaction of 5,6-bis(4-bromophenyl)-3-hydrazino-1,2,4-triazine (61) with some phosphorus reagents in non-polar solvent under various temperatures, the 6,7-diaryl-2,3-dihydro-3,3,3-triphenyl-1,2,4,-triazaphospholino[4,5-b][1,2,4]triazine (62) was synthesized by stirring compound 61 with dibromotriphenylphosphorane in THF at room temperature for 24 hours [Scheme 15]6. Formation of 62 may be occurred via heterocyclization of the intermediate N1,N2-disubstituted hydrazine. The 13CNMR spectrum of compound 62 showed signal at δ 29.61 ppm52.
![Scheme 15. Synthesis of 6,7-diaryl-2,3-dihydro-3,3,3-triphenyl-1,2,4,-triazaphospholino[4,5-b][1,2,4]triazine](http://www.orientjchem.org/wp-content/uploads/2015/02/Vol31_No1_FUSE_Dina_sch15-150x150.jpg) |
Scheme15: Synthesis of 6,7-diaryl-2,3-dihydro-3,3,3-triphenyl-1,2,4,-triazaphospholino[4,5-b][1,2,4]triazine Click here to View scheme |
Phosphorylation of 3-hydrazino-1,2,4-triazine 61 via treatment with acetonyl triphenylphosphonium chloride under stirring in THF-piperidine for 24h, at room temperature furnished N1,N2-disubstituted hydrazine 63. Ring closure of 63 by heating above its melting point produced the heterobicyclic system 64 [Scheme 16]6. On the other hand, that reaction when carried out under refluxing, the isomeric structure 5,6-bis-aryl-3-{(3,3,3-triphenyl)-5-methyl-3,4-dihydro-2H-1,2,3-N5–diazaphosphol-2-yl}-1,2,4-triazine (65) [Scheme 16]6.
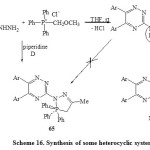 |
Scheme16: Synthesis of some heterocyclic system Click here to View scheme |
the treatment of 3-hydrazinotriazine 61 with diethylphosphite and/or 2-chlorophenyl di-chlorothiophosphate in THF/piperidine at room temperature produced the phosphonohydrazide 66 and phosphono-hydrzidothionic acid 67 respectively [Scheme 17], while the reactions under reflux afforded directly the 1,2,4,3-triazaphospholo[4,5-b][1,2,4]triazines 68 and 69 respectively [Scheme 17]. 1HNMR spectra of 68 and 69 showed signals of NH protons at δ10.92 and 10.34 ppm, respectively with respect to similar reported data53-55.
imilarly, compound 70 isolated from treatment of 61 with diphenyl(2,4,6-trimethylbenzoyl)phosphorus oxide by stirring with THF at room temperature while 7,8-bisaryl-4,4-diphenyl-3-(2`,4`,6`-trimethyl-phenyl)-4H-451,2,4-triazino[3,2-c][1,2,4,5]triaza- phosphinin-4-ol (71) was isolated under refluxing [Scheme 18]6.Due to driving force of P=O bond is strong and phenyl groups are bad leaving groups, the nucleophilic attack of hydrazine moiety may be carried out at carbonyl group then P=O group6.
![Scheme 17. Synthesis of 1,2,4,3-triazaphospholo[4,5-b][1,2,4]triazines 68 and 69](http://www.orientjchem.org/wp-content/uploads/2015/02/Vol31_No1_FUSE_Dina_sch17-150x150.jpg) |
Scheme17:Synthesis of 1,2,4,3-triazaphospholo[4,5-b][1,2,4]triazines 68 and 69 Click here to View scheme |
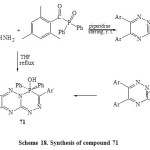 |
Scheme18: Synthesis of compound 71 Click here to View scheme |
Phosphorus compounds with a α-nitrogen or α-oxygen more often exhibited interesting biological properties56. Thus, introducing of P-C-O or P-C-N pattern into heterocyclic systems may be enhancing their biocidal effects. Thus, compounds 62-71 showed strong effects on the tested snails, which due to facile donation and back donation in d-orbital of phosphorus atom6.
Condensation of 4-oxo-4H-chromene-3-carboxaldehyde (72) with phosphonic dihydrazide (1:1 by moles) when heated in boiling ethanol yielded the mono-hydrazone 73. Addition of diethyl phosphite to the azomethine bond of monohydrazone 73 by heating at 80-100ºC in TEA led to the direct formation of 3-(4-amino-5-ethoxy-3,5-dioxido-1,2,4,3,5-triazadiphosphinan-6-yl)4H-chromen-4one(74) [Scheme 19]57, 58.
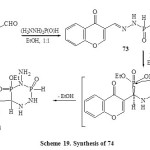 |
Scheme19: Synthesis of 74 Click here to View scheme |
Pnickuk et al59, reported that the intramolecular heterocyclization of polysubstituted pyrazole 75 afforded the fused phospha-heterobicyclic systems 76 via loss of dialkylamine.
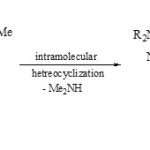 |
Scheme 19A Click here to View scheme |
Functionally 1,2,4,3-triazaphospholopyridines (78) were obtained from [1+4] cycloaddition of 1-amino-2-imino-2H-pyridine-3-carbonitrile (77) with P(NMe2)3 in refluxing benzene60.
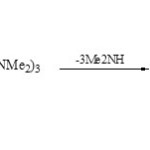 |
Scheme 19B Click here to View scheme |
In 13CNMR spectra these compounds absorb further downfield in the range δ 265-218.
Also, N-isoquinolinium 79 reacts with R-CºP in boiling toluene (glass pressure tube)61 to form the 1,3-azaphospholoquinoline derivatives 80
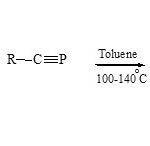 |
Scheme 19C Click here to View scheme |
Under the same conditions, [3+2] cycloaddition of N-cycloimmoni-um ylide 81 with phosphaalkyne (82) has been successfully employed to synthetic of 1,3,4-azaphospholo[1,2-a]Phthalazine (83)61.
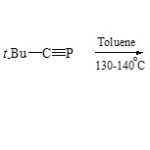 |
Scheme 19D Click here to View scheme |
On the other hand, 2,3-dialkylbenzothiazolium bromides (84) when condense with PCl3/ Et3N led to the direct formation of compound 8562 .
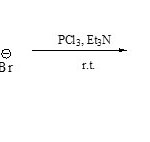 |
Scheme 19E Click here to View scheme |
In addition, 3-alkyl-2-aminobenzthiazolium (86) when react with PCl3/ Et3N yielded the 1,2,4-phosphazabenzthiazole 8763,64.
 |
Scheme 19F Click here to View scheme |
Most spectral study of organophosphorus heterocyclic compounds has been undertaken because of their wide applications in agriculture as pesticide and industry, The mass spectra of a series of 2-substituted-2,3-dihydro-1H-naphtho[1,8-de](1,3,2)-diazaphosphorine-2-oxides (88) were studied to establish their fragmentation processes. The major fragmentation patterns are the loss of aryloxy substituent and RPO2H moieties from the molecular ion. The fragmentation pattern is supported by meta stables, high resolution and collision activation dissociation data [Scheme 20A]61a. Raju et al61 b, reported the synthesis, electron impact and high resolution mass spectral of 2-substituted-3-(4-methylphenyl)-naphth[1,2-e](1,3,2)oxazaphosphorine-2-oxides/sulfide.
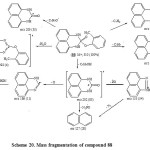 |
Scheme20: Mass fragmentation of compound 88 Click here to View scheme |
Spectrophotometric Determinations of Phosphorus
Numbers of spectrophotometric methods for determination of phosphorus, based on the formation of heteropoly acid with molybdate have been described. The molar absorptivity of these heteropoly acids in the aqueous solution or in organic solvents after solvent extraction, are generally low61. Several cationic dyes, for example; methylene blue, ethyl violet, crystal violet, auramine, malachite green have been used for the determination of phosphorus62. The spectrophotometric determination of phosphates using above dyes processes have limited use due to several disadvantages.63
Recently, A novel curing agent of epoxy resins (EPO), bis(3-amino-2-thienyl) phenylphosphine oxide (ABTPPO), was synthesized and characterized. ABTPPO was used as a flame retardant curing agent, and used to prepare a novel halogen-free flame retardant EPO composite.64 In addition to a reported of a miniature flow-through detector methods useful for bimodal, photometric and fluorimetric, determination of phosphates 65 and A simple manifold flow injection analysis (FIA) for determining phosphorus in the presence of arsenate in water66
Conclusion
This review cover published literature concerning the synthetic of fused biheterocyclic nitrogen containing phosphorus atom over the period 1999-2014, updating our earlier review6 and on the area of bioactive 1,2,4-triazine derivatives11,12,40,67,68, 69.
The developments in this area will help to persuade the important strategy to synthesis of phospha-heterocyclic nitrogen-five and six-membered rings.
References
- M. Balali-Mood, M. Abdollahi (eds.), Basic and Clinical Toxicology of Organophosphorus Compounds, DOI 10.1007/978-1-4471-5625-3, Springer-Verlag London 2014, p 25-34.
- Q. Dai, R. Chen; Phosphorus, Sulfur and Silicon 1997, 122, 261.
- L. Nion, F. C. Ru-Yn Chen, J. A. Zhou, Phosphorus, Sulfur and Silicon 1997, 130, 65.
- J. S. Tang, J. G. Verkade; U S Patent, 5 260 436 1993; Chem. Abstr. 1993, 120, 218836v.
- A. K. Tiwari, B. K. Dubey, I. C. Shukla, J. Indian Chem. Soc. 2003, 80, 717.
- I. C. Shukla, P. K. Dwived, J. Indian Chem. Soc. 2005, 82, 670.
- T. E. Ali, R. M. Abdel-Rahman, F. I. Hanafy; Phosphorus, Sulfur and Silicon 2008, 183, 1-13.
- A. E. Wroblewski, J. Pinkas, J. G. Verkade, Main Group Chem. 1995, 1, 69.
- A. Naiini, V. G. Young, J. G. Verkade; Polyhedron, 1997, 16, 2087.
- P. Mc Laughlin, J. G. Verkade; Organometallics, 1998, 17, 5937.
- B. D. Sa, J. G. Verkade; J. Am. Chem. Soc., 1996, 118(12), 832.
- R. M. Abdel-Rahman; Pharmazie, 2001, 56(1), 18-22.
- R. M. Abdel-Rahman; Pharmazie, 2001, 56(3), 195-204.
- R. M. Abdel-Rahman; Boll. Chim. Farmaceutico, 2001, 140,401.
- Y. Xu, K. Yan, B. Song, G. Xu, S. Yang, W. Xue, D. Hu, P. Lu, L. Ouyang, Z. Chen; Molecule, 2006, 11, 6660.
- L. Wan, I. Alkorta, J. Elguero, J. Sun, W. Zheng; Tetrahedron, 2007, 63, 9129.
- F. W. Krock, F. Krohnke; Chem. Ber., 1969, 102, 659.
- U. Bergstra, A. Hoffmann, M. Regitz; Tetrahedron Lett. 1992, 33, 1049.
- R. H. Huisgen, J. Org. Chem., 1976, 41, 403.
- R. K. Bansal, K. Karaghosoff, N. Gupta, A. Schmidpeter, C. Spindler, Chem. Br., 1991, 124, 475.
- R. K. Bansal, N. Gupta, A. Surana; J. Indian Chem. Soc., 1998, 75, 648.
- R. M. Abdel-Rahman; Trends Heterocycl. Chem., 2002, 8, 187.
- R. K. Bansal, V. Kabra, N. Gupta, K. Karaghiosoff; J. Indian Chem. Soc., 1992, 31B, 254.
- N. Gupta, C. B. Jain, J. Heinnicke, N. Bharatiya, R. K. Bansal, P. G. Jones; Heteroatom Chem., 1998, 9, 333.
- R. K. Bansal, N. Gupta, R. Gupta, G. Pandey, M. Agarwal; Phosphorus, Sulfur and Silicon 1996, 112, 121.
- R. K. Bansal, R. Mahnot, D. C. Sharma, K. Karaghiosoff, A. Schmidpeter; Heteroatom Chem., 1992, 3, 351.
- G. Markl, S. Pflaum; Tetrahedron Lett. 1987, 28, 1511.
- K. Karaghiosoff, R. K. Bansl, N. Gupta; Z. Naturfosch. Teil B. 1992, 47, 373.
- R. K. Bansal, G. Pandey, R. Gupta, K. Karaghiosoff, A. Schmidpeter; Synthesis, 1995¸173.
- G. Markl, S. Pelaum, Tetrahedron Lett., 1988, 29, 3387.
- R. K. Bansal, N. Gandhi, K. Karaghiosoff, A. Schmidpeter; Z. Naturforsch. Teil, 1995, 50, 558.
- K. Karaghiosoff, A. Schmidpeter, P. Mayer “Synthetic Methods in Organometallic Chemistry”. G. Thieme, Stuttgart, 3, Group, 1995.
- K. Karaghiosoff, C. Cleve, A. Schmidpeter; Phosphorus, Sulfur, 1986, 28, 289.
- I. A. Litvinov, K. Karaghiosoff, A. Schmidpeter, E. Y. Zabotina, E. N. Dianora; Heteroatom Chem., 1991, 2, 369.
- E. C. Taylor, I. J. Turchi; Chem. Rev., 1979, 79, 2.
- A. Schmidpeter, G. Jochem; Tetrahedron Lett., 1992, 33, 471.
- P. Molina, M. Alajarin, A. Lopez-Lazaro; J. Chem. Soc., Perk. Trans. 1986, 1(12), 2037 .
- P. Molina, M. Alajarin, A. Lopez-Lazaro; Tetrahedron, 1990, 46(12), 4353.
- Q. Dai, R. Chen; Phosphorus, Sulfur and Silicon 1997, 122, 261.
- J. Zhou, Y.Qui, K. Feng, R-Y. Chen; Synthetic Commn., 1998, 28(24), 4673.
- R. M. Abdel-Rahman; Phosphorus, Sulfur and Silicon 2000, 166, 315.
- P. Molina, M. Alajarin, A. Lopez-Lazaro; Tetrahedron, 1991, 47(33), 6747.
- Y. O. El-Khoshnieh; Phosphorus, Sulfur and Silicon 2000, 139, 163.
- Y. O. El-Khoshnieh, Y. A. Ibrahim; Phosphorus, 1995, 101, 67.
- Z. El-Gendy, J. Morsy, H. Allimony, W. Abdel-Momen, R. M. Abdel-Rahman Phosphorus, Sulfur and Silicon 2003, 178(9), 2055.
- R. Lu, N. Yang, Z. Shang, Phosphorus, Sulfur and Silicon 1996, 108, 197.
- M. R. Mohan, T. S. Hafez, M. M. Henary; Phosphorus, Sulfur and Silicon 1998, 139, 13 .
- T. B. Hwang, L. Feiliu, W.-Qian Yan, X.-Ming Yu, Xu.-Hong Qian, J.-L. Zkang; Phosphorus, Sulfur and Silicon 1997, 122, 299.
- S. N. Tandura, M. G. Voronkov, N. V. Alekseer; Top. Curr. Chem., 1986, 131, 99.
- M. A. H. Laramary, J. G. Verkade, Z. Anorg; Allg. Chem., 1991, 605, 163.
- Y. A. Ibrahim, A. M. Kadry, M. R. Ibrahim, J. N. Lisgarten, B. S. Potfer, R. A. Palmer; Tetrahedron 1999, 55, 13457.
- L. Nian He, R.-XI, Zhuo, X-Peng Liu, F. Caio, Phosphorus, Sulfur and Silicon 1999, 144, 453.
- Y. Haribaba, M. A. Kumar, K. Srinivasulu, C. S. Reddy, C. N. Raju; Arkivoc, 2006, 189.
- M. Maffei, G. Buono, Tetrahedron 2003, 59, 8821.
- M. R. Bryce, R. S. Matthews; J. Organomet. Chem., 1997, 325, 153.
- J. Hernadez, F. M. Gaycoalea, D.Z. Rivera, J. J. Onofre, K. Martinez, J. Lizardi; Tetrahedron, 2006, 62, 2520.
- A. J. Cristu, J. L. Pirat, D. Birieux, J. Monbrum, C. Ciptadi. Y. A. Beko; J. Organomet. Chem., 2005, 690, 2472.
- T. El-Sayed Ali; Arkivoc, 2008, 2, 71-79.
- M. Maffei, G. Buono, Tetrahedron 2003, 59, 8821.
- A. M. Pinhuk, A. S. Merkulov, G. V. Oshovsky, A. A. Tolmachas; Phosphorus, Sulfur and Silicon 1999, 144-146, 713.
- a) C. Devendranath R., G. T. Reddy, C. N. Raju; Indian J. Heterocycl. Chem.., 1995, 4, 285). b. C. N. Raju, C. Das, G. S. Reddy; Indian J. Heterocycl. Chem., 1995, 4, 281.
- C. Wodlin, M. G. Mollon; Anal. Chem., 1953, 25, 1168.
- S. Motomizu, M. Oshima, A. Hirashima; Anal. Chim. Acta., 1988, 211, 119.
- Xu M., Zhao W., Li B., Yang K. and Lin L., Synthesis of a phosphorus and sulfur-containing aromatic diamine curing agent and its application in flame retarded epoxy resins, Fire and Materials. doi: 10.1002/fam.2252, 2014.
- Marta Fiedoruk, Elżbieta Mieczkowska, Robert Koncki, Łukasz Tymecki. A bimodal optoelectronic flow-through detector for phosphate determination. Talanta, 2014, 128(1), 211-214
- V. Gerez, K. Rondano, C. E. L. Pasquali. A simple manifold flow injection analysis for determining phosphorus in the presence of arsenate Journal of Water Chemistry and Technology, 2014, 36(1), 19-24
- S. Chakravarty, R. K. Mishra; J. Indian Chem. Soc., 1994, 71, 717.
- R. M. Abdel-Rahman; Pharmazie, 1999, 54(11), 791.
- R. M. Abdel-Rahman; Pharmazie, 2001, 56(4), 275.

This work is licensed under a Creative Commons Attribution 4.0 International License.









