Synthesis, characterization and antimicrobial screening of some fluorinated chromones and chlorochromones
S. G. Jagadhani*, S. G. Kundalikar and B. K. Karale
P. G. and Research department of Chemistry, Radhabai Kale Mahila Mahavidyalaya, Ahmednagar, 414001, India.
DOI : http://dx.doi.org/10.13005/ojc/300475
Article Received on :
Article Accepted on :
Article Published : 22 Dec 2014
4-Bromo-2-fluorobenzaldehyde when treated with substituted hydroxyacetophenones yields chalcones 3. These chalcones were cyclized using DMSO/I2 gave the compound 2-(4-Bromo-2-fluorophenyl)-4H-chromen-4-one 4. Chalcones on treatment with DMSO/CuCl2 yields 2-(4-bromo-2-fluorophenyl)-3-chloro-4H-chromen-4-one 5. The structures of compounds have been established on the basis of spectral data. These compounds were screened for their antimicrobial activities.
KEYWORDS:Fluorinated Chromones; Chlorochromones; Antimicrobial activity
Download this article as:| Copy the following to cite this article: Jagadhani S. G, Kundalikar S. G, Karale B. K. Synthesis, characterization & antimicrobial screening of some fluorinated chromones and chlorochromones. Orient J Chem 2014;30(4). |
| Copy the following to cite this URL: Jagadhani S. G, Kundalikar S. G, Karale B. K. Synthesis, characterization & antimicrobial screening of some fluorinated chromones and chlorochromones. Available from: http://www.orientjchem.org/?p=6132 |
Introduction
A Fluorinated compound plays a vital role in a chemical and biochemical sciences. As per literature over 10% of newly registered pharmaceutical drugs and some 40% of newly registered agrochemicals contain one or more fluorine atoms.1
Halogeno substituted chromones with heterocyclic substituent at 2-position are reported to have broncho-dilatory2 and coronary-spasmolytic3 properties.
Chalcones and their analogues having an α, β-unsaturated carbonyl system are very versatile substrates for the evaluation of various organic reactions.4
The chalcones were found to have good antibacterial, analgesic and anti-inflammatory activities.5
Compounds containing chromone moiety are synthetically versatile molecules with a reactive carbonyl group having considerable significance for their biological activities6-7 and for their reactivity with nucleophiles, which allow the synthesis of a wide veriety of heterocycles. Chromones are reported to exhibit anti-inflammatory,8 antimicrobial,9 anticancer,10 antiallergic11 and coronary vasodialator12 activity. Some chromones has potential HIV-integrase inhibition activity.13-17 Chromones can be used as fungicides and stimulants for the central nervous system.18
The various methods for the synthesis of 3-halochromones were reported by different co-workers. Gammill19 synthesized 3-halochromone from enaminoketone with halogen containing reagents.
Results and Discussion
2- Hydroxyacetophenones 1 on treatment with fluorinated aldehydes 2 afforded compound 3 chalcones. Chalcone showed IR absorption bands at 3523 cm-1 due to –OH functionality. 1HNMR shows peak at 12.30 δ due to –OH protons. The strucure of compounds were also confirmed by Mass spectrum.
Compound 3 on treatment with DMSO and catalytic amount of I2 yields compound 4 chromones. Compound 4 showed IR absorption band at 1670 cm-1 due to C=O functionality and gives characteristic 1HNMR signals for protons. This structure is also confirmed by Mass spectrum.
Compound 4 on treatment with DMSO and CuCl2 yields compound 5 chlorochromones. Chlorochromones are confirmed by IR frequencies like 1662 cm-1
for C=O and 1108 cm-1 for Ar-Cl. This structure is also confirmed by 1HNMR and Mass spectrum.
Antimicrobial Activity
Synthesized compounds were screened for their antibacterial activities against the bacteria E–coli, B. Subtilis, and A. Niger using standard antibiotic drug Streptomycine. The biological activities of these compounds have been evaluated by turbidity method using DMSO as a solvent. MIC was determined after 48 hr or 72 hr incubation. Incubation zone were measured in mm and results obtained are shown in Table 2. Most of the compound showed moderate and few compound showed good antibacterial activity against E–coli & B. Subtilis.
Experimental
Melting points were determined in open capillary tubes and are uncorrected. IR spectra were recorded on a Perkin-Elmer FT spectrophotometer in a KBr disc. 1H NMR spectra were recorded on a Bruker Avance II 400 MHz spectrophotometer DMSO-d6 as a solvent and TMS as an internal standard (chemical shift in δ values). Mass spectra were obtained on a Finnigan mass spectrometer. Purity of the compounds was checked by TLC on silica gel G plates.
General Procedure
(2E)-3-(4-Bromo-2-Fluorophenyl)-1-(2-Hydroxyphenyl)Prop-2-en-1-one
2-Hydroxyacetophenone (0.005 mol) 1 and aldehyde 2 (0.005mol) were taken in 100 ml RBF with 25 ml ethanol. To this reaction 2 gm of NaOH/KOH was added and resulting reaction was stirred at room temperature for 24-36 hrs. Then contents were poured over crushed ice and acidify with Conc. HCl, solid thus obtained were seperated by filtration and crystallized from proper solvent to get compound 3. Their characterization data is given in the table
Spectral Data
3a: IR(KBr) cm-1: 3523 (-OH strech), 1642 (C=O), 1612-1573 (Ar –C=C- Strech).
1H NMR (DMSO-d6): 2.34 (s, Ar-CH3), 6.86-8.12 (m, 5 Ar-H and ∞-olefenic H, 7.84 β-olefenic H.
Mass: (M+); m/z 335
2-(4-Bromo-2-Fluorophenyl)-4H-Chromen-4-one
Chalcones 3 (0.001 mol) was dissolved in15 ml of DMSO and to this catalytic amount of I2 (about 0.5g) was added. The reaction mixture was then heated under reflux for 2 hr. Cold water was added to the flask and the separated product was filtered and washed with water followed by dil. sodium thiosulphate solution to remove excess of iodine. The product was crystallized from alcohol.
Spectral Data
4a: IR IR(KBr) cm-1: 2925 (Aliphatic C-H strech), 1635(C=O strech), 1132 (Ar-F) . 1H NMR (DMSO-d6): 2.45 (s, Ar-CH3), 6.75 (1H), 7.56-8.20 (m, 6 Ar-H),
Mass: (M+); m/z 334
2-(4-Bromo-2-Fluorophenyl)-3-Chloro-4H-Chromen-4-one
Compound 3 (0.001 mol) was dissolved in 15 mL of DMSO and to this reaction mixture excess of CuCl2 (2 g) was added. The reaction mixture was heated under mild reflux for 3h and left overnight. Then 100 mL of ice cold water was added in it. The solid obtained was filtered and washed with dil. HCl followed by water. The product was crystallized from alcohol. The data of the synthesized compounds is given in Table 1.
Spectral Data
5c: IR (KBr) cm-1: 3075 (Aromatic C-H Strech), 1665 (C=O), 1135 (C-F strech), 1078 (Ar-Cl strech).
1H NMR (DMSO-d6): 2.51 (s, Ar-CH3), 7.63-8.13(m, 5 Ar-H) Mass: (M+); m/z 402
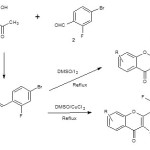 |
Scheme 1 Click here to View scheme |
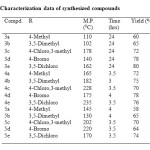 |
Table1: Characterization data of synthesized compounds Click here to View table |
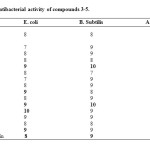 |
Table2: Antibacterial activity of compounds 3-5. Click here to View table |
References
- Cottet F., Marulla M., Lefebvre O., and Shkosser M., Eur J Org Chem., 1559, (2003).
- Wiley P. F., J. Am. Chem. Soc., 74, 4329 (1952).
- a) Jongerbreur G., Arch Intern Pharmacodynamie, 90, 384 (1952). b) Schmtz J., Hirt R., Eichenberger F. E., Lavener H., Helv. Chim. Acta., 36, 620 (1953). c) Wander A. G., Birtish Patent, 27, 728, 767 (1955). d) Koo J., J. Org. Chem., 26, 1635 (1961).
- Kelly D. R., Carroff E., Lood R. W. F., Heal W. and Roberts S. M., Chem Commun, 2016 (2004).
- Sam Solomon W. D., Ravi T. K. and Annadurai K., Indian Drugs, 36(7), 466 (1999).
- Madje B R, Shindalkar S. S., Ware M. N. and Shingare M. S., Arkivoc, 14, 82 (2005).
- Gerwick W. H., Lopez A., Van Duyne G. D., Clardy J., Ortiz W. and Buez A., Tetrahedron Lett, 270, 1986 (1979).
- Inaba T. and Tanaka K., Chem Pharm Bull, 48, 131 (2000).
- Mazzei M., Scottotattori E., Dondero R. and Ibrahim M., II Farmaco, 54, 452 (1999).
- Donnelly D. and Geogphegan R., J Med. Chem., 8, 872 (1965).
- Nohara A., Kuriki H., Saijo T., Sugihara H., Kanno M. and Sannopp Y, J. Med. Chem., 20, 141 (1977).
- Bariana D. S., J. Med. Chem., 12, 927 (1969).
- Gao Y. J., Zhang Z. J. and Xue Q. J, J. Mater. Res. Bull., 34, 19977 (1867).
- Chen S. L., Jix S., Zhu Z. H. and Yao Z. G., Dyes pigment, 23, 275 (1993).
- Jin G. Y., Hou Z, Zhao G. F., Cao C. Y. and Li Y. C., Chem. J. Chin. Univ., 18, 409 (1997).
- Lu S. M. and Chen R. Y., Org. Prep. Proced. Int., 32, 302 (2000).
- Hui X. P., Zhang L. M., Zhang Z. Y., Wang Q. and Wang F., Indian J. Chem., 38(B) , 1066 (1999).
- Franinyuk M. S. and Khilya V. P., Khim. Geterotsikl. Soed., 3 (1999)
- Gammill R. B., Synthesis, 901(1979).

This work is licensed under a Creative Commons Attribution 4.0 International License.









