Nano-silver Colloidal Solution Formation by a Simple and Green Method
Hamid Reza Ghorbani1*
1Department of Chemical engineering, Qaemshahr Branch, Islamic Azad University, Qaemshahr, Iran
DOI : http://dx.doi.org/10.13005/ojc/300364
Article Received on :
Article Accepted on :
Article Published : 01 Sep 2014
Silver nanoparticles were obtained in aqueous medium, at room temperature, by simple and low cost route. The synthesis involves the use of silver nitrate, polyvinylpyrrolidone (pvp), dextrose and water as the silver precursor, stabilizing agent, reducing agent and solvent respectively. In order to identify and analyze nanoparticles, UV – Vis spectroscopy, transitional electron microscopy (TEM), and dynamic light scattering (DLS) were used. All data showed evidence for the formation of silver nanoparticles, with the size of 6–12 nm.
KEYWORDS:Silver Nanoparticles; Green Synthesis; Dextrose; pvp
Download this article as:| Copy the following to cite this article: Ghorbani H. R. Nano-silver Colloidal Solution Formation by a Simple and Green Method. Orient J Chem 2014;30(3). |
| Copy the following to cite this URL: Ghorbani H. R. Nano-silver Colloidal Solution Formation by a Simple and Green Method. Orient J Chem 2014;30(3). Available from: http://www.orientjchem.org/?p=4493 |
Introduction
In recent years, the synthesis and applications of nano particles of noble metals have been of a great interest for researchers. Silver nano particles provide a wide range of valuable opportunities for such fields of science as catalysis, photonics, photography, chemical sensing, surface enhanced Raman scattering (SERS) and most importantly in the medicinal field as anti-microbial agents [1, 2, 3]. These properties extensively depend on the size and shape of the nano material. Synthesis of silver particles is an important topic [4]. Development of methods for nano structures synthesis is necessary to produce nano particles that can be employed in different applications. There are several methods for fabrication of silver nano particles. Generally, silver nano particles can be prepared by chemical methods such as chemical reduction and electro chemical techniques, physical methods such as Arc-discharge and physical vapor condensation and biological methods such as the use of microorganisms [5, 6, 7, 8].
In 2014, spherical Silver nano particles (SNPs) were synthesized by chemical reduction method from a metal precursor silver nitrate in presence of an anionic surfactant and strong reducing agent [9]. In another work, Metallic silver nano particles was reduced from silver nitrate by employing the extra cellular enzymatic machinery of edible White button Mushroom (Agaricus bisporus) [10].Ghorbani reported biosynthesis of silver nano particles using Salmonella typhirium The size of the nano particles was determined to be 87 ± 30 nm, applying dynamic light scattering. [11]. In another paper, the Lens culinaris seed extract was used to green synthesis of silver nanoparticles at temperature of 25°C [12]. A green low-cost and reproducible yeast mediated synthesis of silver nano particles was reported by Jha et al. at room temperature [13]
This paper presents the synthesis of silver nano particles by simple and green route. In this paper, we employed silver nitrate as an initial reagent, dextrose as reducing agent, and polyvinyl pyrrolidone (pvp) as a stabilizer.
Materials and methods
Materials
The silver nitrate as the precursor, Dextrose as the reducing agent and polyvinyl pyrrolidone (PVP) as the stabilizing agent to synthesize nano silver colloids were purchased from Sigma-Aldrich.
Preparation
1 ml of aqueous solution of dextrose (3 g/100 ml) and 1 ml polyvinylpyrrolidone (20 g/l) were added to 100 ml aqueous solution of silver nitrate (0.001 M). The reaction between this solution and Ag+ ions were carried out in bright conditions for 5 min. After 5 min, the colorless solution of silver nitrate in the container turns into brown color. This color change indicates a possibility of nano-silver colloidal solution production.
Characterization of Silver nano particles
Uv-vis spectroscopy was used to prove the existence of nano particles. The morphology and size was determined by the transitional electron microscopy (TEM), and DLS analysis.
Results
For analytical study of the prepared sample, the amount of absorption within wave length of 350 – 600 nm was observed by uv-vis spectroscopy. It is known that an absorption band at about 400–420 nm due to surface plasmon resonance in silver nano particles [14]. Fig. 1 shows the UV-Vis spectra of Nano-silver Colloidal Solution recorded between 350 and 600 nm. As illustrated the SPR band cantered 412 nm confirms the formation of silver nano particles in the solution [15].
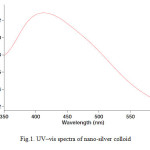 |
Fig1: UV–vis spectra of nano-silver colloid
|
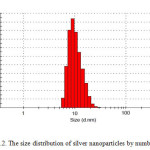 |
Fig2: The size distribution of silver nano particles by number Click here to View Figure |
It is well known that silver can be reduced from Ag+ to Ag0 by dextrose. Dynamic light scattering is a widely used technique for the determination of particle size in colloidal solution. As is seen in figure 2, average size of nano particle synthesized is 9 nm. The distribution of silver nano particles is about 6 nm which indicates narrow distribution of the nano particles. SEM analysis was carried out to understand the topology of silver nano particles. At the end, silver nano particles were studied by transitional electron microscopy (TEM). Fig. 3 confirms silver nano particles formation at nano size.
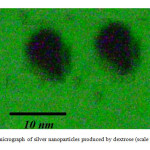 |
Fig3: TEM micrograph of silver nano particles produced by dextrose (scale bar=10 nm)Click here to View Figure |
Conclusions
This is an environment friendly one-step method for synthesis of nano-silver colloid. The dextrose acted as a reducing agent of AgNO3 and polyvinylpyrrolidone (pvp) as a capping material. Nano particles in the range of ~9 nm are synthesized by dextrose when it is added to AgNO3. It seems this method is a fast and green method to form Ag nano partciles. The Ag nano particles were characterized by UV-visible, TEM and DLS analysis.
References
- Prabhu, S.; Poulose, E.K. Int. Nano Lett., 2012, 2, 32.
- Alahmad, A.; Eleoui, M.; Falah, A.; Alghoraib, I. Phys Sci Res Int., 2013, 1(4), 89-96.
- Dong, P. V.; Ha, C. H.; Binh, L. T. Int. Nano Lett., 2012, 2, 9.
- Kasbohm, J. Int J Nanomedicine, 2012, 7, 1543–1550.
- Lanje1, A. S.; Sharma, S. J.; Pode, R. D. J. Chem. Pharm. Res., 2010, 2(3), 478-483.
- Mani, U.; Dhanasingh, S.; Arunachalam, R.; Paul, E.; Shanmugam, O.; Rose, C.; Mandal, A. B. Prog Nanotechnol Nanomater, 2013, 2 (1), 21-25.
- Moazeni, M.; Shahverdi, A. R.; Nabili, M.; Noorbakhsh, F.; Rezaie, S. Nanomedicine Journal, 2014, 1(4), 267-275.
- Mohan, S.; Oluwafemi, O. S.; George, S. C.; Jayachandran, V. P.; Lewu, F. B.; Songca S. P.; Kalarikkal, N.; Thomas, S. Carbohydr Polym, 2014, 15, 469-474.
- Ghorbani, H. R.; Safekordi, A. A.; Attar, H.; Rezayat Sorkhabadi, S. M. Chem. Biochem. Eng. Q., 2011, 25 (3,) 317–326.
- Zambare; A.; Nerpagar, T.; Chaudhari, N.; Manchalwad, P.; Harke, S. Int. J. Nano Dimens., 2014, 5(6), 569-573.
- Papaiah, S.; Seshadri Goud, T. E.; Devi Prasad, B. S.; Narasimha, G.; Vemana, K. Int. J. Nano Dimens., 2014, 5(2), 139-144.
- Ghorbani, H.R. J. Nanostructure Chem., 2013, 3, 29.
- Shams, S.; Pourseyedi, S. H.; Hashemipour Rafsanjani, H. Int. J. Nanosci Nanotechnol, 2014, 10(2), 127-132.
- Jha; A. K.; Prasad, K.; Kulkarni, A. R. Int. J. Nanosci Nanotechnol, 2008, 4(1), 17-22.
- Wang, Q.; Mao, P. F. Int. J. Nano Dimens, 2013, 4(2), 167-170.
- Mohammadinejad; R.; Pourseyedi, S. H.; Baghizadeh, A.; Ranjbar, S. H.; Mansoori, G. A. Int. J. Nanosci Nanotechnol, 2013, 9(4), 221-226.

This work is licensed under a Creative Commons Attribution 4.0 International License.









