Catalytic Depolymerization and Kinetics of Polyethelene Terepthalate by Saponification
Dilip B. Patil1, Vijendra Batra2and Sushil B. Kapoor3
1Dept. of Chemistry, Institute of Science, Nagpur-440001 (India)
2Dept. of Chemistry, SarvodayaMahavidyalaya, Sindewahi, Chandrapur-441222 (India)
3Dept. of Chemistry, Art, Commerce and Science College, Tukum, Chandrapur-442402 (India)
DOI : http://dx.doi.org/10.13005/ojc/300338
Article Received on :
Article Accepted on :
Article Published : 10 Feb 2015
Conductometric measurement technique has been deployed to study kinetic of depolymerization of polyethylene terephthalate (PET) and NaOH using cobalt acetate and manganese acetate catalyst. Chemical kinetic of this reaction shows second order kinetics. The specific reaction rate without catalyst is 2.88 X 10-3g-1 s-1 at 70oC. Specific reaction rates were determined at various temperatures ranging from 50oC to 80oC. From the data, Arrhenious constant, energy of activation were determined and found to be 5.0 X 106min-1 and 54.2 KJg-1 respectively. Similarly the activation parameters were determined using cobalt acetate and manganse acetate as catalyst.
KEYWORDS:Kinetics; catalysis; saponification; conductometry; PET waste
Download this article as:| Copy the following to cite this article: Patil D. B, Batra V, Kapoor S. B. Catalytic Depolymerization and Kinetics of Polyethelene Terepthalate by Saponification. Orient J Chem 2014;30(3). |
| Copy the following to cite this URL: Patil D. B, Batra V, Kapoor S. B. Catalytic Depolymerization and Kinetics of Polyethelene Terepthalate by Saponification. Orient J Chem 2014;30(3). Available from: http://www.orientjchem.org/?p=7096 |
Introduction
Number of methods of recycling of PET are available in the literature1-4. For PET saponification much research has been carried out in acidic environment. Pusztaseri5 studied saponification in sulphuric acid. Sato and Yoshiaka6 also studied saponification under acidic conditions. Alcoholysis has been carried out by different workers7-13. Acid, alkali and water saponification of PET in organic solvent have been reported by several workers14-18.
In present work, alkali saponification of PET waste powder is under taken using cobalt acetate and manganese acetate as a catalyst. A rapid online conductivity measurement are carried out to study the kinetics. In the kinetic study, specific reaction rates were determined with and without catalyst. The energy of activation, Arrhenious constant were determined for saponification of PET waste powder.
Experimental
All chemical used in the present work were of analytic reagent grade. The solution of sodium hydroxide was prepared using conductivity water. PET waste bottles were procured from local corporation area of Nagpur, Maharashtra, India. The bottles were washed with Teepol and then with double distilled water. All the bottles were dried with hot air blower. The dried bottles were chilled to increase its brittleness, then crushed, ground and sieved into different particle sizes ranging from 100µm to 800µm.
The optimum parameters for saponification of PET waste powder were determined by gravimetric measurement of product terepthalic acid (TPA). All kinetics measurements were carried at optimum parameters (Table 1). In kinetic measurements, three vertical neck round bottom flask was fixed with refluxed water condenser, an internal digital temperature measurement probe, a conductivity measuring cell and a micro-controller based vertical type stirrer. A digital conductivity monitoring instrument made of Equiptronic India Ltd. was used to measure conductivity. High precision thermostat and digital temperature measurement probe was used in the present work.
 |
Table1: Kinetics of saponification of PET waste Powder: Optimum parameters Click here to View table |
In kinetic experiment, 10g PET waste powder (100µm) and 3 ml pyridine was added into reaction flask placed in the thermostat. Conductivity cell and tip of temperature measurement probe was adjusted so that they are not struck by vertical stirrer bar. 7g of sodium hydroxide in 100ml of water was added to PET waste powder containing pyridine. Immediately stop-watch was started and conductivity measured at various time intervals up to 150 min. From specific conductivity of water (Cw),specific conductivity of reaction mixture at various time(Ct),specific conductivity at zero time (C0)and specific conductivity at infinity (C∞), the values of (Co-Ct)/(Ct –C∞) were determined at various time interval. From plot of Co-Ct/Ct-C∞versus time, specific reaction rate were determined.
In order to evaluate kinetic and thermodynamic parameters, specific reaction rate determinations, in presence and absence of catalyst were determined at various temperatures ranging from 50oC to 80oC. From results, energy of activation, frequency factor were evaluated.
Results and Discussion
Specific conductivity of unreacted sodium hydroxide solution in the reaction system at various intervals of time were determined. A plot of Co-Ct/Ct–C∞ versus time is plotted and found to be linear. It indicates the second order nature of this saponification reaction. The slope of the plot gives the specific reaction rate, k2 (Table 2 and Fig. 1). The variation of specific reaction rate, k2, with the amount of sodium hydroxide and PET waste powder, confirms the second order kinetic of saponification.
 |
Table2: Kinetics of Depolymerisation of PET waste powder Click here to View table |
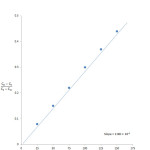 |
Figure1: kinetics of depolymerisation of PET waste powder: Click here to View figure |
The activation parameters of depolymerisation of PET waste powder by saponification were evaluated by measuring the specific reaction rate at various temperature ranging from 50oC to 80oC. The specific reaction rate varies from 0.88X10-3g-1s-1 to 5.12X10-3g-1s-1.
From results, a plot of log k2 versus 1/T is plotted and it gives straight line (Table 3 and Fig. 2). From slope of this plot, the energy of activation is evaluated and found to be 54.2 KJg-1. The frequency factor is 5X106 min-1.
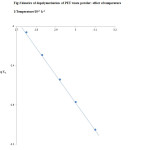 |
Figure2: kinetics of depolymerisation of PET waste powder: effect of temperature Click here to View figure |
 |
Table3: Kinetics of Depolymerisation of PET waste powder: effect of temperature Click here to View table |
The addition of small amount of manganese acetate shows a remarkable effect in enhancing the specific reaction rate ofdepolymerization of PET waste powder. The specific reaction rate increases from 0.88 X10-3g-1s-1 to 1.60 X10-3g-1s-1 at 50oC. The increase in temperature from 50oC to 80oC, increases the specific reaction rate from 1.60 X10-3g-1s-1 to 4.08 X10-3g-1s-1.
A plot of log k2 versus 1/T is linear and the values of energy of activation, in presence of manganese acetate is 28.4 KJg-1. which is lower than in absence of added manganese acetate i.e. 54.2 KJg-1 (Table 4 and Fig. 3). All these facts strongly suggest that the manganese acetate has a catalytic effect on depolymerization of PET waste powder.
 |
Table4: Kinetics of Depolymerisation of PET waste powder: effect of temperature in presence of manganese acetate Click here to View table |
 |
Figure3: Kinetics of depolymerisation of PET waste powder: effect of temperature in presence of manganese acetate Click here to View figure |
Similarly, addition of cobalt acetate increases the specific reaction rate from 0.88 X10-3 g-1s-1 to 1.68 X10-3g-1s-1 at 50oC. Increase in temperature from 50oC to 80oC increases the specific reaction rate from 1.68 X10-3g-1s-1 to 4.40 X10-3g-1s-1. A plot of log k2 versus 1/T gives energy of activation as 27.6 KJg-1. which is also lower than that in absence of cobalt acetate. It indicates that cobalt acetate also has catalytic effect on depolymerization of PET waste (Table 5 and Fig. 4)
 |
Table5: Kinetics of Depolymerisation of PET waste powder: effect of temperature in presence of cobalt acetate Click here to View table |
 |
Figure4: Kinetics of depolymerisation of PET waste powder: effect of temperature in presence of cobalt acetate Click here to View figure |
References
- Erlenbach, E.H. ; Laundenbach, E.S. and Obenburg, R.L.; U.S. Pat.,1962,03 ,037/050
- Kuroda, Y.;Yamaguchi, R. and Matsumoto,R.; Japan kokai Pat.,1973, 48,68538
- Miura, K.; Kagiya, Y. and Ichikawa, T.; Japan, Kokai Pat., 1968, 6823,449
- Baliga, S. and Wong, T. W.; J.Polym. Sci. part A., polym chem., 1989,27,2701
- Pusztanseri, S.F., U.S. Pat.,1987, 27,2701
- Yashioka, T. ; Sato, T.andOkuwaki,A., J Appl. polym. Sci.,1994,52,1353-1355 Ref. 4
- Vaidya,V.R. and Nadkarni,V. M., Ind. Engng. Chem. Res., 1998, 27,2056
- Vaidya, V.R. and Nadkarni,V. M., Ind. Engng. Chem. Res., 1987,26, 194
- Orekov, V.N. and Rudenko, B.M., Vest Khar’kpolytech Inst., 1982 , 195,10
- Sniezko, A.; Penczek, P. and Ostrysk, R., InstIndChemwarsaw pol., Forbe lack, 1981,87, 1014,
- Leaversuch, R.D., Morden plastic, 1991,68,41
- Mikalojezyk, B.; Lubawy, A.; Djewska, M.; Smoczynski, P., Poziniak A., Boebel, 11, Pol. Pat PL, 1985, 120,009
- Yashioka, T.; Sato, T.; Okuwaki A., J. Appl. PolymSci , 1994, 52,1353
- Companelli, J.R.; Kamal, M.R.; Cooper, D.G., Appl. Polym Sci.,1993, 48,443
- Yoshioka,T.;Okayama,N.andOkuwaki,A., Ind. Engng. Chem. Res,1998,37,336-340
- Yoshioka, T.; Motoki, T. and Okuawki, A., Ind. Engng. Chem. Res,2001,40,75-79
- Mishra,S.; Goje,A.S.; and Zope,V.S., International conference on plastic waste Management and Environment, proceeding, New Delhi, 2001, 163-169

This work is licensed under a Creative Commons Attribution 4.0 International License.









