Synthesis and antimicrobial activity of novel Indol compounds containing 2-azitidinones and 1,3,4 oxadiazoles
J. Sreeramulu*1 , P. Ashokgajapathiraju1
Department of chemistry , S.K.University , Anantapuramu, A.P, India.
DOI : http://dx.doi.org/10.13005/ojc/300234
Article Received on :
Article Accepted on :
Article Published : 23 May 2014
New novel derivatives of 4-(3-(1-((4-acetyl-5-methyl-5-(p-substituted phenyl)-4,5-dihydro-1,3,4-oxadiazol-2-yl)methyl)-5-chloro-1H-indol-3-yl)-1-(pyridin-4-yl)-1H-pyrazol-4-yl)-3-chloro-1-(4 substituted phenyl)azetidin-2-one (5a-g) were prepared by the condensation of acetohydrazide (4a-g) with acetic anhydride. The compound 4(a-g) was obtained by the reaction of (3) with 4-substituted acetophenone in the presence of glacial aceticacid. The synthon (3) was obtained by the reaction of compound(2) with hydrazine hydrate in ethanol. The compound (2) was obtained by the reaction of (1H-indol-1-yl)acetate(1) with monochloroacetyl chloride in the presence of triethylamine in dioxane. The structure of the newly synthesized compounds were charecterized by IR, NMR, Mass and elemental analysis.
KEYWORDS:1;3;4-oxadiazole; acetic anhydride; acetophenone; monochloro acetyl chloride; antimicrobial activity.
Download this article as:| Copy the following to cite this article: Sreeramulu J, Ashokgajapathiraju P. Synthesis and antimicrobial activity of novel Indol compounds containing 2-azitidinones and 1,3,4 oxadiazoles. Orient J Chem 2014;30(2). |
| Copy the following to cite this URL: Sreeramulu J, Ashokgajapathiraju P. Synthesis and antimicrobial activity of novel Indol compounds containing 2-azitidinones and 1,3,4 oxadiazoles. Orient J Chem 2014;30(2) Available from: http://www.orientjchem.org/?p=3436 |
INTRODUCTION
1,3,4,-Oxadiazoles are five membered heterocycles having two nitrogen atoms and one oxygen atom. 1,3,4-oxadiazoles belong to the group of heterocycles that have been attracting attention for last two decades due to their wide range of biological interactions. Some 1,3,4-oxadiazoles substituted witharyl groups at positions 2 and 5 are of significant interest of polymer and material sciences because of their electro chemical properties(Phosphorescence). 1,3,4-oxadiazole derivatives have played a major role in the pharmaceutical chemistry.Literature reveals that a large number of heterocyclic compounds containing the 1, 3, 4-Oxadiazoles ring are associated with diverse pharmacological properties such as anti-inflammatory, antimicrobial, fungicidal and antiviral activity[1-4]. Substituted 1,3,4-oxadiazole have revealed antibacterial [5,6], antitubercular [7],vasodialatory [8], antifungal [9,10], cytotoxic [11], anti-inflammatory and analgesic [12,13,14,15], hypolipidemic [16], anticancer [17,18] and ulcerogenic [19] antimycobacterail[20], anticonvulsant [21] activities. We have designed and synthesized new 1,3,4-Oxadiazoles as a potential antimicrobial agent. The results of this study are discussed in this paper.
MATERIALS AND METHODS
All the chemicals used in the present investigation were purchased from Ark pharma, Inc. and Sigma-Aldrich Chemicals company, Inc.USA. and used without further purification. TLC was performed on aluminium sheet of silica gel 60F254,E-Merk,Germany using iodine as visualizing agent. Melting points were determined in open capillary tubes on Mel-Temp apparatus and are uncorrected(in degree celsius) .Columnchromatography was performed on silica gel with different solvent systems as eluents to afford the pure compound. The Infra Red Spectra of the compounds were recorded in KBr pellets on FT-IR(perkin-Elmer 1000 units)instrument . All 1H and13C-NMR spectra were recorded on a varian XL-300 Spectrometer operating at 400MHz for 1H-NMR and 75 MHz for 13C-NMR. The 1H-NMR spectra were recorded using TMS as an internal standard(Chemical shifts in δppm). The compounds were dissolved in DMSO-d6 and Chemical shifts were referenced to TMS (1H and 13C-NMR). Mass spectral data was recorded on FAB-MS instrument at 70ev with direct inlet system. Elemental analysis were recorded on a Carlo Erba 1108 elemental Analyser, Central Drug Research Institute, Lucknow, India.
Preparation of Intermediates
2-(5-chloro-3-(4-(3-chloro-4-oxo-1-(4- substituted phenyl)azetidin-2-yl)-1- (pyridin-4-yl)-1H-pyrazol-3-yl)-1H-indol-1-yl)-N’-(1-(p-substitutedphenyl)ethylidene)acetohydrazide (4a-g)
A mixture of 2-(5-chloro-3-(4-(3-chloro-4-oxo-1-(4- substituted phenyl)azetidin-2-yl)-1-(pyridin-4-yl)-1H-pyrazol-3-yl)-1H-indol-1-yl)acetohydrazide(3) (1.62mmol,1g) in hot methanol (10ml), acetophenone (10 mmol) and a drop of glacial acetic acid were added. Resulting reaction mixture was refluxed for 3hrs at room temperature.After completion of the reaction as indicated by TLC. The solid that separated was filtered wash with cold methanol and purified by column chromatography by using hexane:ethylacetate(7:3) used as eluent to afford 2-(5-chloro-3-(4-(3-chloro-4-oxo-1-(4-(trifluoromethyl) phenyl) azetidin-2-yl)-1
(pyridin-4-yl)-1H-pyrazol-3-yl)-1H-indol-1-yl)-N’-(1-phenylethylidene) acetohydrazide(4a). The reaction procedure leding to (4a) , was then extended to 4(b-g) from (3) reaction with 4-methyl,4-methoxy,4-chloro,4-bromo,4-nitro,4-trifluoromethyl acetophenone to afforded the compounds 4(b-g).
Synthesis of Compounds
Ethyl 2-(5-chloro-3-(4-(3-chloro-4-oxo-1-(4-substitutedphenyl)azetidin-2-yl)-1- (pyridin-4-yl)1H- pyrazol-3-yl)-1H-indol-1-yl)acetate (2)
A mixture of Schiff’s Base ethyl2-(5-chloro-3-(1-(pyridin-4-yl)-4-(((4-(trifluoromethyl) phenyl) imino)methyl)-1H-pyrazol-3-yl)-1H-indol-1-yl)acetate (1) (20mmol ,11.03g) in acetone, triethylamine (0.005mol, 0.75 ml) was added. To this, a solution of chloroacetyl chloride (30 mmol, 3.5 ml) was added drop wise with stirring. The Mixture was refluxed up to 8h. The triethyl amine hydrochloride formed was filtered and washed several times with acetone. The filtrate and washings were mixed and concentrated under reduced pressure. The residue obtained was washed with petroleum ether (40-600C) to remove the unreacted Schiff’s base and the solid obtained was recrystallized from ethanol to afford compound(2).
2-(5-chloro-3-(4-(3-chloro-4-oxo-1-(4-substitutedphenyl)azetidin-2-yl)-1-(pyridin-4-yl)1H -pyrazol-3-yl)-1H-indol-1-yl)acetohydrazide (3)
A mixture of Ethyl 2-(5-chloro-3-(4-(3-chloro-4-oxo-1-(4-substitutedphenyl)azetidin-2-yl)-1- (pyridin-4-yl)-1H-pyrazol-3-yl)-1H-indol-1-yl)acetate (2) (20mmol) and hydrazine hydrate (30mmol) in ethanol 20ml was refluxed for 6-7hours. The reaction mixture was cooled and poured on to ice cold water with stirring. The progress of the reaction was monitored by TLC with hexane:ethyl acetate(7:3) as elutent. The separated solid was filtered, washed with water and recrystallized from ethanol to afford compound (3).
4-(3-(1-((4-acetyl-5-methyl-5-(p-substituted phenyl)-4,5-dihydro-1,3,4-oxadiazol-2-yl) methyl)-5-chloro-1H-indol-3-yl)-1-(pyridin-4-yl)-1H-pyrazol-4-yl)-3-chloro-1-(4-substitu ted phenyl)azetidin-2-one (5a-g)
A mixture of 4a (1mmol,716.54mg ) and excessive acetic anhydride (10ml) was refluxed for 3hrs.The progress of the reaction was monitored by TLC. The excessive acetic anhydride was distilled off and the residue was poured on to crushed ice. The solid thus obtained was filtered and purified by column chromatography by using hexane:ethylacetate (7:3) as eluent to afford compound(5a) (493.07 mg, 0.65mmol). The above cyclisation reaction was then extended to synthesize 5(b-g) from 4(b-g) reaction with acetic anhydride.
RESULTS AND DISCUSSION
The target compounds were synthesized via the route as shown in the Scheme.The synthon required for the synthesis of the target molecules was prepared by a reported method, filtered and recrystallized from ethanol. For all the synthesized compounds, the progress of the reaction was monitored by TLC with cyclohexane, ethylacetate (7:3) as mobile phase. All the synthesized structures showed satisfactory result. The chemical shift values of the synthesized compounds were full agreement with the number of protons present in it.
Scheme 1
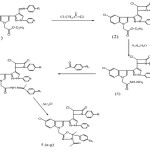 |
Scheme 1 Click here to View Scheme |
Synthetic route for target compounds
|
Compound |
5a |
5b |
5c |
5d |
5e |
5f |
5g |
|
R1 |
-H |
-CH3 |
-OCH3 |
4-Cl |
4-Br |
4-NO2 |
-CF3 |
|
R |
-CF3 |
-CF3 |
-CF3 |
-CF3 |
-CF3 |
-CF3 |
-CF3 |
The structures of the newly synthesized compounds were supported by physical data (Table-1) and following spectral analysis.
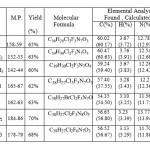 |
Table 1 Click here to View table |
Physical, Analytical and Spectral data for the synthesized title compounds are given as follows.
Characterization of 2-(5-chloro-3-(4-(3-chloro-4-oxo-1-(4-(trifluoromethyl)phenyl) azetiding-2-yl)-1-(pyridin-4-yl)-1H-pyrazol-3-yl)-1H-indol-1-yl)-N’-(1phenylethylidene) acetohydrazide (4a)
yield 65%, M.P:162-630C, IR (KBR) : (δppm) 3190 cm-1( –NH), 3041 cm-1(=CH), 1696 cm-1(C=O), 1625cm-1(C=N), 677cm-1(C-Cl) respectively. 1H-NMR (400MHz ,DMSO-d6) δppm: 10.90(s,1H,-CONH),8.10(s,1H,Pyrazole),7.75-8.40(m,4H of -C5H4N) 7.30-7.70(m,4H,-CH of indol),6.80-7.20(m,9H, of -C6H5 and C6H4CF3), 5.43(d,1H,-CH of azitidin attached to -Cl), 5.10(d, 1H,CH of azitidin ring),3.65 (s,2H, N-CH2-CO), 2.25(s,3H, N-CH3). Mass(m/z) : 715.15 , Anal.Calcd.For C28H20Cl2F3N7O2 : C, 60.34%; H, 3.66%; N, 13.68%; O, 4.47% . Found: C 60.15%, H 3.41%, N 13.57%, O 4.25% .
Characterization of 2-(5-chloro-3-(4-(3-chloro-4-oxo-1-(4-(trifluoromethyl)phenyl) azitidin -2-yl)-1-(pyridin-4-yl)-1H-pyrazol-3-yl)-1H-indol-1-yl)N’-(1-(p-tolyl)ethylidene) acetohydra zide (4b)
yield 60 %, M.P: 157-58 0C, IR (KBR) : (δppm) 3185 cm-1( –NH), 3042cm-1(=CH), 1685 cm-1(C=O), 1625 cm-1(C=N), 676cm-1(C-Cl) respectively. 1H-NMR (400MHz ,DMSO-d6) δppm: 10.89 (s,1H,-CONH),8.09(s,1H,Pyrazole),7.75-8.40(m,4H of -C5H4N), 7.30-7.70(m,4H,-CH of indol),6.85-7.15(m,8H, of -C6H4 and C6H4CF3) ,5.42(d,1H,-CH of azitidin attached to -Cl), 5.11 (d,1H,CH of azitidin ring),3.60(s,2H,N-CH2-CO), 2.30(s,3H,N-CH3),1.52(s, 1H,-CH3).
Mass(m/z) : 729.16 , Anal.Calcd.For C37H28Cl2F3N7O2 : C, 60.83%; H, 3.86%; N, 13.42%; O, 4.38%. Found: C 60.62%, H 3.54%, N 13.21%, O 4.17% .
Characterization of 2-(5-chloro-3-(4-(3-chloro-4-oxo-1-(4-(trifluoromethyl)phenyl) azetidin-2-yl)-1-(pyridine-4-yl)-1H-pyrazol-3-yl)-1H-indol-1-yl)N’-(1-(4-methoxyphenyl) ethylidene )acetohydrazide (4c)
yield 63%, M.P: 148-49 0C, IR (KBR) : (δppm) 3184 cm-1( –NH), 3040cm-1(=CH), 1680cm-1(C=O), 1620 cm-1(C=N), 675 cm-1(C-Cl) respectively. 1H-NMR(400MHz ,DMSO-d6)δppm:10.85 (s,1H,-CONH),8.07(s,1H,Pyrazole),7.75-8.40(m,4H of -C5H4N),7.30-7.70 (m, 4H,-CH of indol),6.80-7.16(m,8H, of -C6H4 and C6H4CF3) 5.40(d,1H,-CH of azitidin attached to –Cl), 5.10 (d,1H,CHof azitidin ring),3.55(s,2H, N-CH2-CO),2.32(s,3H, N-CH3),3.85 (s,1H,-OCH3).Mass(m/z) : 745.16 , Anal.Calcd.For C37H28Cl2F3N7O3 : C, 59.53%; H, 3.78%; N, 13.13%; O, 6.43%. Found: C 59.34%, H 3.57%, N 12.95%, O 6.26% .
Characterization of 2-(5-chloro-3-(4-(3-chloro-4-oxo-1-(4-(trifluoromethyl)phenyl) azetidin -2-yl)-1-(pyridin-4-yl)-1H-pyrazol-3-yl)-1H-indol-1-yl)-N’-(1-(4-chlorophenyl) ethylidene)acetohydrazide (4d)
yield 67%, M.P: 168-690C, IR (KBR) : (δppm) 3200 cm-1( –NH), 3045cm-1(=CH), 1690cm-1(C=O), 1623 cm-1(C=N), 676 cm-1(C-Cl) respectively. 1H-NMR(400MHz ,DMSO-d6)δppm: 10.92 (s,1H,-CONH),8.08(s,1H,Pyrazole),7.75-8.40(m,4H of -C5H4N),7.30-7.70(m,4H,-CH of indol),6.85-7.18(m,8H, of -C6H4Cland C6H4CF3) 5.44(d,1H,-CH of azitidin attached to –Cl) ,5.10 (d,1H,CH of azitidin ring), 3.57 (s,2H, N-CH2-CO),2.31(s,3H, N-CH3). Mass(m/z) : 749.11 , Anal.Calcd.For C36H25Cl3F3N7O2 : C, 57.58%; H, 3.36%; N, 13.06%; O, 4.26% , Found: C 59.42%, H 3.18%, N 12.88%, O 4.01% .
Characterization of N’-(1-(4-bromophenyl)ethylidene)-2-(5-chloro-3-(4-(3-chloro-4-oxo-1-(4-(trifluoromethyl)phenyl)azetidin-2-yl)-1-(pyridin-4-yl)-1H-pyrazol-3-yl)-1H-indol-1-yl) acetohydrazide (4e)
yield 66%, M.P:165-670C, IR (KBR) : (δppm)3205cm-1(-NH),3044cm-1(=CH), 1687cm-1 (C=O) ,1623 cm-1(C=N), 677 cm-1(C-Cl) respectively. 1H-NMR(400MHz ,DMSO-d6) δppm: 10.91(s,1H,-CONH),8.09(s,1H,Pyrazole),7.75-8.40(m,4H of -C5H4N),7.30-7.70(m,4H,-CH of indol), 6.80-7.20(m,8H, of -C6H4Brand C6H4CF3) 5.43(d,1H,-CH of azitidin attached to –Cl), 5.10 (d,1H,CH of azitidin ring), 3.58 (s,2H, N-CH2-CO),2.32(s,3H, N-CH3). Mass(m/z) : 793.06 , Anal.Calcd.For C36H25 BrCl2F3N7O2 : C, 54.36%; H, 3.17%; N, 12.33%; O, 4.02 % . Found: C 59.16%, H 3.02%, N 12.15%, O 3.83% .
Characterization of 2-(5-chloro-3-(4-(3-chloro-4-oxo-1-(4-(trifluoromethyl)phenyl) azetidin-2-yl)-1-(pyridin-4-yl)-1H-pyrazol-3-yl)-1H-indol-1-yl)-N’-(1-(4-nitrophenyl) ethylidene)acetohydrazide (4f)
yield 68 %, M.P: 186-87 0C, IR (KBR):(δppm) 3215cm-1(-NH), 3047cm-1(=CH),1695cm-1(C=O) ,1626cm-1(C=N), 678 cm-1(C-Cl) respectively. 1H-NMR(400MHz ,DMSO-d6)δppm:10.95 (s, 1H,-CONH),8.10(s,1H,Pyrazole),7.75-8.40(m,4H of -C5H4N),7.30-7.70(m,4H,-CH of indol) ,6.95-7.28(m,8H,of-C6H4NO2 and C6H4CF3),5.44(d,1H,-CH of azitidin attached to –Cl) ,5.09 (d,1H,CH of azitidin ring),3.69 (s,2H, N-CH2-CO),2.30(s,3H, N-CH3). Mass(m/z) : 760.13 , Anal.Calcd.For C36H25Cl2F3N8O4 : C, 56.78%; H, 3.31%; N, 14.71%; O, 8.40%. Found: C 56.63%, H 3.16%, N 14.58%, O 8.24% .
Characterization of 2-(5-chloro-3-(4-(3-chloro-4-oxo-1-(4-(trifluoromethyl)phenyl) azetidin-2-yl)-1-(pyridine-4-yl)-1H-pyrazol-3-yl)-1H-indol-1-yl)-N’-(1(4(trifluoromethyl) phenyl) ethylidene)acetohydrazide (4g)
yield 70%,M.P:175-76 0C,IR(KBR): (δppm) 3210 cm-1( –NH), 3046cm-1(=CH), 1694cm-1(C=O),1624cm-1(C=N),676cm-1(C-Cl)respectively.1H-NMR (400MHz, DMSO-d6) δppm :10.93 (s,1H,-CONH),8.081(s,1H,Pyrazole),7.75-8.40(m,4H of -C5H4N),7.30-7.70(m,4H,-CH of indol),6.90-7.25(m,8H, of two -C6H4CF3 rings), 5.45(d,1H,-CH of azitidin attached to –Cl) ,5.11 (d,1H,CH of azitidin ring),3.65 (s,2H, N-CH2-CO),2.35(s,3H, N-CH3). Mass(m/z) : 783.14 , Anal.Calcd.For C37H25Cl2F6N7O2 : C, 56.64%; H, 3.21%; N, 12.50%; O, 4.08%. Found: C 56.48%, H 3.07%, N 12.35%, O 3.92% .
Table 2
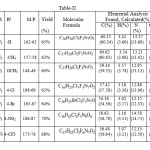 |
Table 2 Click here to View table |
Characterization of 4-(3-(1-((4-acetyl-5-methyl-5-phenyl-4,5-dihydro-1,3,4-oxadiazol-2-yl)methyl)-5-chloro-1H-indol-3-yl)-1-(pyridin-4-yl)-1H-pyrazol-4-yl)-3-chloro-1-(4(trifluoro methyl) phenyl)azetidin-2-one (5a)
yield 65%, M.P:158-590C, IR (KBR) : (δppm) 3042cm-1(=CH(aromatic),),1698cm-1 (C=O) ,1620cm-1(C=N), 1140 cm-1(N-N), 678 cm-1(C-Cl) respectively. 1H-NMR (400MHz, DMSO-d6) δppm : 8.10(s,1Hof Pyrazole),7.75-8.43(m,4H of C5H4N) 7.30-7.70(m,4H,-CH of indol),6.80-7.25(m,9H, of -C6H5 and C6H4CF3), 5.45(d,1H,-CH of aziti din attached to -Cl), 5.15(d,1H,-CH of azitidin ring),3.55(s,2H,-NCH2 attached to indol nucleus), 2.46(s,3H of -COCH3 group), 2.22(s,3H,-CH3). C13-NMR 400MHz , DMSO-d6 (δ ppm) : 129, 111,121, 126, 123, 113, 135, 130, 126,129,116, 61, 62, 162, 143, 134, 125,132,124,147, 114, 150, 60, 159, 90, 169, 24, 28, 142, 127, 128.5, 126.5 corresponding to C1, C2, C3, C4, C5, C6, C7, C8, C9, C10, C11,C12,C13,C14, C15, C16&C20, C17 &C19,C18,C21,C22,C23&C26,C24 &C25, C27, C28, C29, C30, C31, C32,C33,C34&C38,C35 &C37 and C36carbon atom respectively. Mass(m/z) : 757.16 , Anal.Calcd.For C38H28Cl2F3N7O3 : C, 60.17%; H, 3.72%; N, 12.93%; O, 6.33%. Found: C 60.02%, H 3.67%, N 12.78%, O 6.17% .
Characterization of 4-(3-(1-((4-acetyl-5-methyl-5-(p-tolyl)4,5-dihydro-1,3,4-oxadiazol-2-yl)methyl)-5-chloro-1H-indol-3-yl)-1-(pyridin-4-yl)-1H-pyrazol-4-yl)-3-chloro-1-(4-(trifluoro methyl) phenyl)azetidin-2-one (5b)
yield 64 %, M.P:152-530C, IR (KBR) : (δppm) 3042cm-1(=CH(aromatic),),1695cm-1(C=O),1620cm-1 (C=N), 1645&1232 cm-1(1,3,4-oxadiazole), 676cm-1(C-Cl) respectively. 1H-NMR (400MHz, DMSO-d6) δppm : 8.09(s,1H,N-CH gp.),7.75-8.41(m,4Hof C5H4N),7.30-7.70(m,4H,-CH of indol),6.85-7.15(m,8H, of -C6H4 and C6H4CF3) ,5.44(d,1H,-CH of azitidin attached to –Cl), 5.13(d,1H,-CH of azitidin ring),3.52(s,2H,-NCH2 attached to indolnucleus ),2.45(s,3H of -COCH3 group),2.23(s, 3H,CH3) ,1.57(s,3H,-CH3 attached to phenyl ring).. C13-NMR 400MHz DMSO-d6 (δ ppm) : 129.1, 111.2, 121.3, 125.9, 123, 112.7, 134.7, 130, 126, 129, 115.8,61, 62, 162.1 ,142.8, 134, 125.1, 132, 124, 147, 114, 150, 60, 158.2, 90, 168.5, 24, 28, 139,127,128.8,136.5,21. 21.5 corresponding to C1, C2, C3, C4, C5, C6, C7, C8, C9, C10, C11, C12, C13,C14, C15, C16&C20, C17&C19, C18, C21,C22,C23&C26,C24 &C25, C27, C28, C29, C30, C31, C32, C33,C34&C38,C35 &C37, C36 and C39carbon atom respectively. Mass(m/z) : 771.17 , Anal. Calcd. For C39H30Cl2F3N7O3 : C, 60.63%; H, 3.91%; N, 12.69%; O, 6.21% . Found: C 60.47%, H 3.76%, N 12.54%, O 6.04%
Characterization of 4-(3-(1-((4-acetyl-5-(4-methoxyphenyl)-5-methyl-4,5-dihydro-1,3,4-oxadiazol-2-yl)methyl)-5-chloro-1H-indol-3-yl)-1-(pyridin-4-yl)-1H-pyrazol-4-yl)-3-chloro-1-(4-(trifluoromethyl)phenyl) azetidin-2-one (5c)
yield 62 %, M.P:142-440C, IR (KBR) : (δppm) 3042cm-1(=CH(aromatic),),1680cm-1(C=O),1617cm-1 (C=N), 1645&1232 cm-1(1,3,4-oxadiazole), 675cm-1(C-Cl) respectively. 1H-NMR (400MHz, DMSO-d6) δppm: 8.07(s,1H,N-CH gp.),7.75-8.40(m,4Hof C6H4N),7.30-7.70(m,4H,-CH of indol),6.80-7.16(m,8H, of -C6H4 and C6H4CF3), 5.43(d,1H,-CH of azitidin attached to –Cl), 5.12(d,1H,-CH of azitidin ring), 3.50(s,2H,-NCH2 attached to indol nucleus), 2.46(s,3H of -COCH3group), 2.24(s, 3H,-CH3),3.82(s,3H,-OCH3). C13-NMR 400MHz DMSO-d6 (δ ppm) : 129.2, 111, 121.9, 125.5, 122.6,113, 134.5,130,125.5, 129, 116, 61, 62, 162.5, 143, 133.9, 125.5,132,124.3,147,114, 150, 58, 160, 91, 169, 23.9, 28, 135, 128,114.5,158.7,55.8 corresponding toC1, C2, C3, C4, C5, C6, C7,C8,C9,C10,C11,C12,C13,C14, C15, C16&C20, C17 & C19, C18, C21, C22, C23&C26,C24 &C25, C27, C28, C29, C30, C31, C32, C33, C34&C38,C35 &C37, C36 and C39carbon atom respectively. Mass(m/z) : 787.17 , Anal.Calcd.For C39H30Cl2F3N7O4 : C, 59.40%; H, 3.83%; N, 12.43%; O, 8.12%. Found: C 59.24%, H 3.67%, N 12.28%, O 7.98% .
Characterization of 4-(3-(1-((4-acetyl-5-(4-chlorophenyl)-5-methyl-4,5-dihydro-1,3,4-oxadiazol-2-yl)methyl)-5-chloro-1H-indol-3-yl)-1-(pyridin-4-yl)-1H-pyrazol-4-yl)-3-chloro-1-(4-(trifluoromethyl) phenyl)azetidin-2-one (5d)
yield 66 %, M.P:165-670C, IR (KBR) : (δppm) 3042cm-1(=CH(aromatic),),1690cm-1(C=O),1623cm-1 (C=N), 1645&1232 cm-1(1,3,4-oxadiazole), 677cm-1(C-Cl) respectively. 1H-NMR (400MHz, DMSO-d6) δppm: 8.08(s,1H,N-CH gp.),7.75-8.42(m,4Hof C6H4N),7.30-7.70(m,4H,-CH of indol),6.85-7.18(m,8H, of -C6H4Cland C6H4CF3),5.43(d,1H, -CH of azitidin attached to -Cl), 5.13(d,1H,-CH of azitidin ring), 3.57(s,2H,-NCH2 attached to indol nucleus), 2.47(s,3H of COCH3 group), 2.24(s,3H,-CH3). C13-NMR 400MHz DMSO-d6 (δ ppm) : 129.2, 111.3, 121.7, 125.6, 122.4, 112.4, 134.5, 130.3, 125.4, 129, 115.9, 60.9, 62.1, 162.3, 142.8, 133.9, 125.4, 124.1, 146.9, 113.9, 149.9, 60,158.3, 90.2, 168.5, 23.7, 27.9, 140.7, 125.4,128.7,132.3corresponding to C1, C2,C3, C4, C5, C6, C7, C8, C9, C10, C11, C12, C13, C14,C15, C16&C20, C17&C19, C18, C21, C22,C23&C26,C24 &C25, C27, C28, C29, C30, C31, C32, C33, C34&C38,C35 &C37and C36carbon atom respectively. Mass(m/z) : 791.12 , Anal.Calcd.For C38H27Cl3F3N7O3 : C, 57.55%; H, 3.43%; N,12.36%; O,6.05% .Found: C 57.40%, H 3.28%, N 12.21%, O 5.90%
Characterization of 4-(3-(1-((4-acetyl-5-(4-bromophenyl)-5-methyl-4,5-dihydro-1,3,4-oxadiazol-2-yl)methyl)-5-chloro-1H-indol-3-yl)-1-(pyridin-4-yl)-1H-pyrazol-4-yl)-3-chloro-1-(4-(trifluoromethyl) phenyl)azetidin-2-one (5e)
yield 67 %, M.P:162-630C, IR (KBR) : (δppm) 3042cm-1(=CH(aromatic),),1688cm-1(C=O),1623cm-1 (C=N), 1645&1232 cm-1(1,3,4-oxadiazole), 676cm-1(C-Cl) respectively. 1H-NMR (400MHz, DMSO-d6) δppm: 8.09(s,1H,N-CH gp.),7.75-8.42(m,4Hof C6H4N),7.30-7.70(m,4H,-CH of indol),6.80-7.20(m,8H, of -C6H4Brand C6H4CF3),5.42(d,1H, -CH of azitidin attached to –Cl), 5.12(d,1H,-CH of azitidin ring), 3.58(s,2H,-NCH2 attached to indol nucleus), 2.46(s,3H of –COCH3 group) ,2.23(s,3H,-CH3). C13-NMR 400MHz , DMSO-d6 (δ ppm ): 129, 111.2, 121.6, 125.6, 122.4, 112.5, 134.5, 130.2, 125.5, 129,115.9 ,60.9, 62, 162.3, 142.8, 133.8, 125.3, 132.2, 124,147, 114,150, 59,158, 90.2,168.5, 23.9,28, 141.7, 129.2, 131.5, 121.2corresponding toC1, C2,C3,C4, C5, C6, C7, C8, C9, C10,C11,C12,C13,C14, C15, C16&C20, C17&C19, C18, C21, C22,C23 & C26, C24 &C25, C27, C28, C29, C30, C31, C32, C33, C34& C38,C35&C37 and C36carbon atom respectively. Mass(m/z) : 835.07 , Anal.Calcd.For C38H27BrCl2F3N7O3 : C, 54.50%; H,3.25%;N,11.71%; O, 5.73 % .Found: C 54.35%, H 3.10%, N 11.56%, O 5.57%.
Characterization of 4-(3-(1-((4-acetyl-5-methyl-5-(4-nitrophenyl)-4,5-dihydro-1,3,4 oxadiazol -2-yl)methyl)-5-chloro-1H-indol-3-yl)-1-(pyridin-4-yl)-1H-pyrazol-4-yl)-3-chloro -1-(4-(trifluoromethyl)phenyl)azetidin-2-one (5f)
yield 70 %, M.P:184-860C, IR (KBR) : (δppm) 3042cm-1(=CH(aromatic),),1697cm-1(C=O),1625cm-1 (C=N), 1645&1232 cm-1(1,3,4-oxadiazole), 678cm-1(C-Cl) respectively. 1H-NMR (400MHz, DMSO-d6) δppm: 8.10(s,1H,N-CH gp.),7.75-8.45(m,4Hof C6H4N),7.30-7.70(m,4H,-CH of indol),6.95-7.28(m, 8H, of-C6H4NO2 and C6H4CF3),5.44(d, 1H, -CH of azitidin attached to –Cl), 5.14(d,1H,-CH of azitidin ring),3.71(s,2H,-NCH2 attached to indol nucleus), 2.46(s,3H of –COCH3group) , 2.20(s,3H,-CH3). C13-NMR 400MHz , DMSO-d6 (δ ppm ): 129.2, 111.3, 121.7, 125.7, 122.5, 112.5, 134.6, 130.2, 125.4, 129, 115.8, 61, 62.1, 162.2, 142.8, 133.8, 125.3,132.2,124.2,146.9, 113.9, 149.9, 60, 158.2, 90.2, 168.7, 23.8, 27.9, 148.7,127.8,123.7,145.9 corresponding to C1, C2, C3, C4, C5, C6, C7,C8,C9,C10,C11,C12,C13,C14, C15, C16&C20, C17&C19, C18, C21, C22, C23 & C26,C24 &C25, C27, C28, C29, C30, C31, C32, C33, C34&C38,C35 &C37 and C36carbon atom respectively. Mass(m/z) : 802.14 , Anal.Calcd.For C38H27Cl2F3N8O5 : C, 56.80%; H, 3.39%; N, 13.94%; O, 9.96%. Found: C 56.65%, H 3.23%, N 13.77%, O 9.80%.
Characterization of 4-(3-(1-((4-acetyl-5-methyl-5-(4-(trifluoromethyl)phenyl)-4,5-dihydro-1,3,4-oxadiazol-2-yl)methyl)-5-chloro-1H-indol-3-yl)-1-(pyridin-4-yl)-1H-pyrazol-4-yl)-3-chloro-1-(4-(trifluoromethyl) phenyl)azetidin-2-one(5g)
yield 68 %, M.P:178-79 0C, IR (KBR) : (δppm) 3042cm-1(=CH(aromatic),),1698cm-1(C=O),1624cm-1 (C=N), 1645&1232 cm-1(1,3,4-oxadiazole), 677cm-1(C-Cl) respectively. 1H-NMR (400MHz, DMSO-d6) δppm: 8.08(s,1H,N-CH gp.),7.75-8.44(m,4Hof C6H4N),7.30-7.70(m,4H,-CH of indol),6.90-7.25(m,8H, of two -C6H4CF3 rings), 5.45(d,1H, -CH of azitidin attached to –Cl), 5.14(d,1H,-CH of azitidin ring), 3.68(s,2H,-NCH2 attached to indol nucleus), 2.47(s,3H of –COCH3group), 2.18(s,3H,-CH3).C13-NMR 400MHz ,DMSO-d6 (δ ppm): 129.3,111.4,121.7,125.5,122.4,112.5, 134.6, 130.2, 125.4, 129, 115.8, 60.9, 62, 162.2, 142.8,133.8, 125.3, 132.1, 124.1, 146.9, 113.9, 149.9, 60, 158.2, 90.2, 168.5, 23.7, 27.9, 145.9,127.2,124.9,129,124.2 corresponding to C1, C2, C3, C4, C5, C6, C7, C8, C9, C10 ,C11, C12, C13,C14,C15,C16 & C20,C17 & C19,C18,C21,C22,C23 & C26,C24&C25, C27,C28,C29, C30,C31,C32, C33,C34&C38,C35&C37,C36 andC39 carbonatom respectively. Mass(m/z):825.15 , Anal. Calcd. For C38H27Cl2F6N7O3 : C, 56.67%; H, 3.29%; N, 11.86%; O, 5.81%. Found: C 56.52%, H 3.13%, N 11.70%, O 5.65%.
Scheme 2
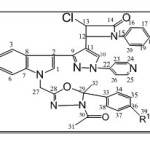 |
Scheme 2 Click here to View Scheme |
Biological activity
The newly synthesized compounds 4-(3-(1-((4-acetyl-5-methyl-5-(p-substituted phenyl)-4,5-dihydro-1,3,4-oxadiazol-2-yl)methyl)-5-chloro-1H-indol-3-yl)-1-(pyridin-4-yl)-1H-pyrazol-4 -yl)-3-chloro-1-(4-substituted phenyl)azetidin-2-one (5a-g), were screened for their antimicrobial studies against antibacterial and antifungal activity by Disc Diffusion method22. The synthesized compounds were used at the concentration of 250μg/ml and 500 μg/ml using DMF as a solvent 23. The amoxicillin 10 μg/disc and cefaclor 30 μg/disc were used as a standard .Whatman No.1 filter paper disk of 5mm diameter were sterile nutrient agar at 45oC,
The sterile disks were impregnated with different compounds synthesized compounds (250μg/ml). The impregnated disks were placed on the medium suitably spaced apart and the plates were incubated at 25 oC for 1 h. To permit good diffusion and then transferred to an incubator at 37 oC for 48 h.for bacteria , and at 28 oC for 72 h. For yeast and fungi. The incubation zones aused by the various compounds on the microorganisms were examined. The results of the preliminary screening test are listed in table-3.
Antibacterial activity
The antibacterial activity of 5(a-g) were screened against the Staphylococus aureus(gram positive), Bacillus cerus, Escherichia Coli (gram negative) and Pseudomonas aeruginosa organisms. In a given series of compounds having nitro (5f) and trifluoromethyl (5g) exhibit high bacterialactivity24,25 when compared to other substituents. The structural activity relationship for different substituents is in the order i.e. –NO2 > -CF3 > -Cl > -Br > -H > -CH3 > -OCH3 . Here amoxicillin and cefaclor are tested as reference compounds to compare the activity. The antibacterial activity of 5(a-g) was shown in the below given table.
Antifungal activity
The antifungal activity of final compounds 5(a-g) were screened aginst aspergillus niger ,Candida albicans . In a given series of compounds containing trifluoro methyl and nitro groups in their structures has shown increased effect on their antifungal activity . The structural activity relationship for different substituents is in the order i.e. –NO2 > -CF3 > -Cl > -Br > -H > -CH3 > -OCH3 Here ketoconazole is tested as reference compound to compare the antifungal activity. Antifungal activity of 4-(3-(1-((4-acetyl-5-methyl-5-(p-substituted phenyl)-4,5-dihydro-1,3,4-oxadiazol-2-yl)methyl)-5chloro-1H-indol-3-yl)-1-(pyridin-4-yl)-1H-pyrazol-4-yl)-3-chloro-1-(4-substituted phenyl) azetidin -2-one (5a-g) was shown in the below given table.
Table 3
| S.NO. | Compd. | Zone of Inhibition (mm) | |||||
| Anti bacterial activity | Anti fungal activity | ||||||
| StaphylococusaureusNCCS 2079 | BacillusCereusNCCS2106 | EscherichiaColiNCCS2065 | Pseudomonas aeruginosa NCCS2200 | Aspergillus nigerNCCS 1196 | Candida albicansNCCS 3471 | ||
| 1) | 5a | 11 | 10 | 11 | 11 | 13 | 15 |
| 2) | 5b | 10 | 09 | 10 | 09 | 12 | 13 |
| 3) | 5c | 08 | 08 | 09 | 08 | 11 | 11 |
| 4) | 5d | 13 | 12 | 11 | 13 | 14 | 19 |
| 5) | 5e | 12 | 11 | 10 | 12 | 13 | 16 |
| 6) | 5f | 17 | 16 | 15 | 16 | 19 | 21 |
| 7) | 5g | 16 | 14 | 13 | 15 | 18 | 20 |
| 8) | Amoxicillin | 21 | 27 | 24 | 22 | ——— | ——– |
| 9) | Cefaclor | 19 | 22 | 19 | 20 | ——— | ——– |
| 10) | Ketoconazol | ——– | ——— | ———– | ———— | 23 | 26 |
Conclusion
Indol bearing pyrazole ring, besides azitidinone moiety and the 1,3,4-oxadiazole group were prepared by acetic anhydride reaction with acetohydrazid group. These synthons were purified & charecterized by chromatographic and spectral techniques. Indol derivatives were subjected to antimicrobial evaluation and some of these compounds were found to posses good anti bacterial and anti microbial activity.
Acknowledgements
The author (P.Ashokgajapathiraju) thanks to U G C – S A P and U G C – B S R , New Delhi for financial assistance and also thankful to IICT Hyderabad and CDRI Lucknow for spectral and analytical data. I express my sincere thanks to my research Supervisor Prof. J.Sreeramulu for his valuable guidance .
References:
- A.Walser, T. Flynn, C. Mason, J. Heterocyclic. Chem. 28 (1991) 1121-1125.
- N.F. Ewiss, A.A. Bahajaj, E.A. Elsherbini, j. Heterocyclic chem. 23 (1986) 1451-1458.
- A.R. Bhat, G.V.Bhat, G.G. Shenoy, J. Pharm. Pharmacol. 53 (2001) 267-272.
- O.G. Todoulou, A.E. Papadaki-Valaraki, E.C.Flippatos, S.llkeda, E.De Clercq, Eur. J. Med. Chem.. 29 (1994) 127-131.
- B.S. Holla, R. Gonsalves, S. Shenoy, Eur. J. Med. Chem. 35 (2000) 267-271. ChemInform Abstract: Synthesis and Antibacterial Studies of a New Series of 1,2- Bis(1,3,4-oxadiazol-2-yl)ethanes and 1,2-Bis(4-amino-1,2,4-triazol-3-yl)ethanes.
- S.F. Barbucenu; G. Bancescu; O.D. Cretu; C. Draghici; A. Bancescu; M. Radu-Popescu. Rev. Chem. (Bucuresti).61.Nr.2., 2010, 140-145.
- G.V.S. Kumar; Y. Rajendraprasad; B.P. Mallikarjuna; S.M. Chandrashekar; C. Kistayya. Eur. J. Med. Chem., 2010, 45, 2063-2074.
- P.J. Shirote; M.S. Bhatia. Arab. J. Chem., 2010, doi: 10.1016/j.ara bjc.2010.07.004.
- O. Parkash; M. Kumar; C. Sharma; K.R. Aneja. Eur. J. Med. Chem., 2010,doi:10.1016 / j.ejmech.2010.06.023.
- X.Z. Jou, L.H.Lai, G.Y.Jin, J.Agrc. Food.Chem.50 (2002) 3757-3760.
- V. Padmavathi; G.S. Reddy; A. Padmaja; P. Kondaiah; Ali-Shazia. Eur. J. Med. Chem., 2009, 44, 2106-2112.
- M. Akhter; A. Husain; B. Azad; M. Ajmal. Eur. J. Med. Chem., 2009, 44, 2372-2378.
- G.A. Idrees; O.M. Aly; G. El-Din; A.A. Abuo-Rahma; M.F.R. Shazia. Eur. J. Med. Chem.,2009, 44, 3973-3980.
- E. Palaska, G. Sahin, P.Kelicen, N.T. Durlu, G. Altinok, II Farmaco 57 (2002) 101-107..
- M.Amir, K. Shikha, Eur.J. Med. Chem. 39 (2004) 535-545.
- B. Jayashankar; K.M.L. Rai; N. Baskaran; H.S.S. Shazia. Eur. J. Med. Chem., 2009, 44, 3898-3902.
- D. Kumar; S. Sundaree; E.O. Johnson; K. Shah. Bioorg. Med. Chem. Lett., 2009, 19, 4492-4494.
- J.J, Bhat, B.R.Shah, H.P.Shah, P.B.Trivedi, N.K. Undavia, N.C. Desai, Indian j. chem 33b (1994) 189-192.
- D. Shashikan; V. Bhandari; K.G. Bothara; M.K. Raut; A.A. Patil; A.P. Sarkate and V.J. Mokale. Bioorg. Med. Chem. Lett., 2008, 16, 1822-1831
- F. Macaev, G. Rusu, S.Pogervnoi, A. Gudima, E. Stingasi, L.Vlad. et.al Bio org. Med. Chem. 13 (2005) 4842-4850.
- A. Zargahi, A. Sayyed, M. Tabatabai, A. Faizi, P. Ahadian, V. Navavi, A. Zanganeh, Shafiee, Biorg. Med.Chem. 15 (2005) 1863-1865.
- G. Nagalakshmi; Indian Journal of Pharmaceutical Science, 2008, plaintiff. 49-55.
- Sudhir Bharadwaj, Bharat Parashar, NarendraParashar and V.K.Sharma, Scholar Research Library, Achieves of Applied Science Research, 2011, 3(2), 558-567.
- J G Colle, J P Duguid, A G Fraser and B P Mammion, “Mackie and McCartne practical Medical Microbiology”, Churchil, Livingston Ltd, London, 13th ed ,Vol.2.1989.
- Irfan Ali Mohammed, Der Pharmacia Sinica, 2011, 2 (6), 102-106.

This work is licensed under a Creative Commons Attribution 4.0 International License.









