Chlorophyll as a simple, inexpensive and environment-friendly colorimetric indicator for NO2 gas
May Kristine O. Bernardo,Voltaire G. Organo*
Department of Physical Sciences and Mathematics, College of Arts and Sciences, University of the Philippines, Manila 1000 Philippines
DOI : http://dx.doi.org/10.13005/ojc/300206
Article Received on :
Article Accepted on :
Article Published : 28 Jun 2014
Chlorophyll is utilized as a simple, inexpensive and environment-friendly (“green”) colorimetric indicator for nitrogen dioxide (NO2) gas. A drastic color change from green to yellow was observed when chlorophyll, either dissolved in CH2Cl2 solution or absorbed into paper, was exposed to NO2 gas. Other gases such as CO2 and SO2 did not exhibit any color change with chlorophyll. Spectroscopic analysis showed nitration of chlorophyll as possible cause for the color change.
KEYWORDS:Chlorophyll; , inexpensive; friendly colorimetric
Download this article as:| Copy the following to cite this article: Bernardo M. K. O, Organo V. G. Chlorophyll as a simple, inexpensive and environment-friendly colorimetric indicator for NO2 gas. Orient J Chem 2014;30(2). |
| Copy the following to cite this URL: Bernardo M. K. O, Organo V. G. Chlorophyll as a simple, inexpensive and environment-friendly colorimetric indicator for NO2 gas. Orient J Chem 2014;30(2). Available from: http://www.orientjchem.org/?p=4062 |
INTRODUCTION
Nitrogen dioxide (NO2) is one of the prevalent oxide species of nitrogen in the atmosphere collectively known as NOx. It is characterized by a reddish-brown color with a pungent irritating odor. This pollutant gas is produced from combustion of fossil fuels and industrial processes. It is also a component of photochemical smog and responsible for production of acid rain. Its accumulation poses a threat to the environment because NO2 is highly reactive, corrosive and oxidative, causing discoloration and degradation of plant species.1 Its presence in the troposphere can also lead to various health issues. At low concentrations, NO2 can induce cough, nasal and eye irritations. At higher concentrations, it can lead to serious respiratory infections.2 These environmental and health concerns due to extensive circulation of NO2/NOx in the atmosphere necessitates development of novel approaches for its detection and monitoring.
In the last decade, many studies have been devoted to selectively monitor the levels of NO2 in the atmosphere. These include metallic oxides,3–6 organic semiconductors,7–9 metal complexes,10,11 and cage-like molecules.12–17 These approaches however require expensive materials and lengthy procedures for its synthesis. Here, we report a very simple and cheap approach towards sensing of NO2 gas by using a common pigment naturally occurring in plants: chlorophyll.
Chlorophyll is a metalloporphyrin pigment responsible for the green color of plants. It plays a major role in photosynthesis. However, chlorophyll is greatly affected by pollutant gases. Chapados and co-workers have observed that chlorophyll reacts with gases such as SO2, H2S, NO and NO2,18 while Guidi et al noted that it can interact with ozone.19 Bevilaqua and co-workers also suggested that chlorophyll can potentially bind CO2 gas based on density functional calculations.20 These studies provide possibilities for chlorophyll as gas sensor. However, application of this natural pigment for naked-eye detection of pollutant gases has not been explored.
EXPERIMENTAL
All reagents and chemicals used were analytical grade and purchased from commercial sources. Solvents were used without any purification.
Extraction of chlorophyll
100 g of cogon grass (Imperata cylindrica) was grinded and macerated for 3-5 min. in ethyl acetate. The crude sample was filtered, placed in a shaker for 5 min. then left overnight at room temperature. The extract was rotavaped to a final volume of 200 mL and carefully decanted into an evaporated dish to exclude any undissolved matter. The extract was then heated to dryness in a water bath at 40°C. The residue was purified using column chromatography (30 g of silica gel with ethyl acetate). The first eluate which was yellow in color was discarded. Methanol was then used to elute the purified extract. The eluate was air dried, resulting to a dark green solid product (0.0395 g). The green product was dissolved with CH2Cl2 and used throughout the experiment.
Production of gases
Sulfur dioxide (SO2) was prepared by adding 10 drops of 2 M HCl to 0.1 g NaHSO3 in a test tube. Nitrogen dioxide (NO2) was prepared by reacting a small piece of copper metal with concentrated HNO3. Carbon dioxide (CO2) was produced by reacting 0.1 g CaCO3 with 10 drops of 5M HCl. The gases were introduced into 3.0 mL chlorophyll solutions via a 5-mL syringe.
Titration of chlorophyll solution
NO2-CH2Cl2 solution was prepared by carefully bubbling NO2 gas to dry CH2Cl2. Concentration of NO2 was determined according to published procedure.21 0-900 mL of NO2-CH2Cl2 solution was used in the titration.
Gas detection using test strips
Filter paper strips (5 cm x 1.5 cm) were soaked in a 5-ml chlorophyll-CH2Cl2 solution until dark green strips were formed. Strips were air dried, transferred in a vial and covered. Gas was introduced into each vial using a syringe.
Spectral Analysis
Spectral scans of untreated and gas-treated chlorophyll solutions in CH2Cl2 were recorded on a Shimadzu 1700 spectrometer from 300-800 nm. Infrared spectra were recorded on a KBr pellet and analyzed using a Perkin-Elmer 1600 series Fourier Transform Spectrophotometer.
RESULTS AND DISCUSSION
Chlorophyll, a green pigment in plants, was investigated for its potential use as colorimetric indicator for NO2. Chlorophyll was extracted from cogon grass using ethyl acetate, purified using column chromatography, and then dried. The CH2Cl2 solution of chlorophyll was placed in a sealed vial and bubbled with NO2 gas. This resulted to a drastic color change of the chlorophyll solution from green to yellowish-brown (Fig. 1). Other gases such as CO2 and SO2 did not exhibit any color change. Previous studies have shown that CO2 has no effect on the structure of chlorophyll, while SO2 gas does not react with chlorophyll in the absence of water.18,22
Fig. 1 Visual detection of gas samples in solution. A) chlorophyll solution in CH2Cl2, B) chlorophyll solution after exposure to CO2, C) chlorophyll solution after exposure to NO2, D) chlorophyll solution after exposure to SO2.
 |
Fig1: Visual detection of gas samples in solution. A) chlorophyll solution in CH2Cl2, B) chlorophyll solution after exposure to CO2, C) chlorophyll solution after exposure to NO2, D) chlorophyll solution after exposure to SO2. Click here to View Figure |
Visible spectra of chlorophyll exposed to NO2 show prominent bands that are distinct from the bands observed in chlorophyll alone (Fig. 2A). Absorption bands with λmax at 414 nm and 666 nm shifted to 424 nm and 679 nm, respectively. The band around 470-480 nm also disappeared upon addition of NO2. These spectral changes are consistent with a study conducted by Chapados et al. showing the effect of NO2 gas on chlorophyll a multilayer.18
Fig.2 (A) UV-Vis spectra of chlorophyll bubbled with NO2 gas. (B) UV-Vis titration of chlorophyll with NO2-CH2Cl2 solution. Insert: Plot of change in absorbance at 414 nm versus NO2 concentration.
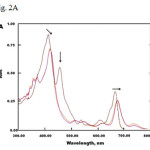 |
Fig 2A: UV-Vis spectra of chlorophyll bubbled with NO2 gas. (B) UV-Vis titration of chlorophyll with NO2-CH2Cl2 solution. Insert: Plot of change in absorbance at 414 nm versus NO2 concentration. Click here to View Figure |
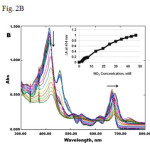 |
Fig2 A: UV-Vis spectra of chlorophyll bubbled with NO2 gas. (B) UV-Vis titration of chlorophyll with NO2-CH2Cl2 solution. Insert: Plot of change in absorbance at 414 nm versus NO2 concentration. Click here to View Figure |
The same spectral change was recorded when chlorophyll was titrated slowly with NO2-CH2Cl2 solution (Fig. 2B). It was also noted that the chlorophyll solution was able to detect NO2 with concentrations greater than 2 mM in CH2Cl2 solution (Fig. 2B insert). Similar titration experiments have been conducted by Rudkevich et al. on calixarenes with NO2–CH2Cl2 solution to estimate limit of detection for NO2.16
Additionally, bubbling oxygen gas into the NO2-treated chlorophyll solution did not change the spectra. This suggests that there is a strong interaction or irreversible reaction that took place between chlorophyll and NO2 gas. Thin layer chromatography of the product formed from the reaction of chlorophyll and NO2 suggests a complete transformation of chlorophyll into a new compound, possibly a nitrated product. This was confirmed by FTIR spectroscopy where distinct bands were detected at 1555 cm-1 and 1372 cm-1 characteristic of an –NO2 group (Fig. 3). Another band at 1282 cm-1 which was not present at the spectrum of chlorophyll solution may be due to formation of an N-NO2 group.23 This result suggests that nitration occurred when chlorophyll was exposed to NO2. Nitration reactions are known to occur in porphyrin complexes caused by NO2 gas.24
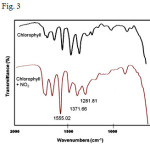 |
Fig3: Portion of FTIR spectra of chlorophyll before and after NO2 exposure. Click here to View Figure |
Interaction between gases and chlorophyll was also studied in solid state using test strips. Figure 4 shows green coloration of filter paper after soaking it in chlorophyll solution. When the dried test strip was exposed to NO2, the color turned from green to yellow-brown, similar to the color change observed in solution. No color change was noted when test strips were exposed to CO2 and SO2. This observation clearly indicates the selectivity of chlorophyll towards NO2 gas. The study also demonstrates as proof-of-principle the potential use of chlorophyll-based sensors for naked-eye detection of NO2 gas.
 |
Fig4: Gas detection using test strips. A) Test strip with chlorophyll alone, B) Test strip after exposure to CO2, C) Test strip after exposure to NO2, D) Test strip after exposure SO2. Click here to View Figure |
CONCLUSION
In conclusion, a simple and cheap colorimetric indicator for NO2 gas based on chlorophyll was developed. This pigment can be obtained easily from plant materials and be used either in solution or as test strips.
Moreover, the method presented here is inexpensive since gases can be produced from common laboratory reagents, and the set-up requires minimal use of equipment. Thus, this method can be performed in the classroom to demonstrate not only the sensitivity of chlorophyll towards NO2 but also the destructive effect of NO2 gas to chlorophyll pigment of plants. Further work will be directed towards improving the detection limit and applying it as NO2 sensor for environmental monitoring.
ACKNOWLEDGMENT
Financial support was provided by the Creative Research and Scholarship Program of the University of the Philippines System.
REFERENCES
- U. S. Environmental Protection Agency, http://www.epa.gov/iaq/no2.html (Accessed December 2013).
- Romieu I., Moreno-Macias H., and London S. J., Proc. Am. Thorac. Soc., 7, 116–122 (2010).
- Chougule M. A., Sen S., and Patil V. B., Ceram. Int., 38, 2685–2692 (2012).
- Afzal A., Cioffi N., Sabbatini L., and Torsi L., Sensors Actuators B Chem., 171-172, 25–42 (2012).
- Kaur J., Roy S. C., and Bhatnagar M. C., Sensors Actuators B Chem., 123, 1090–1095 (2007).
- Tan J., Wlodarski W., and Kalantarzadeh K., Thin Solid Films, 515, 8738–8743 (2007).
- Valentini L., Armentano I., Kenny J. M., Cantalini C., Lozzi L., and Santucci S., Appl. Phys. Lett., 82, 961 (2003).
- Christensen W. H., Sinha D. N., and Agnew S. F., Sensors Actuators B Chem., 10, 149–153 (1993).
- Das A., Dost R., Richardson T., Grell M., Morrison J. J., and Turner M. L., Adv. Mater., 19, 4018–4023 (2007).
- Pochekailov S., Nožár J., Nešpůrek S., Rakušan J., and Karásková M., Sensors Actuators B Chem., 169, 1–9 (2012).
- Shu J. H., Wikle III H. C., and Chin B. A., in ECS Trans., 33, 131–140 (2010).
- Kang Y., Zyryanov G. V., and Rudkevich D. M., Chem. Eur. J., 11, 1924 – 1932 (2005).
- Rudkevich D. M., Eur. J. Org. Chem., 3255–3270 (2007).
- Hines J. H., Wanigasekara E., Rudkevich D. M., and Rogers R. D., J. Mater. Chem., 18, 4050 (2008).
- Organo V. G., Sgarlata V., Firouzbakht F., and Rudkevich D. M., Chem. Eur. J., 13, 4014–23 (2007).
- Kang Y. and Rudkevich D. M., Tetrahedron, 60, 11219–11225 (2004).
- Ohira S.-I., Wanigasekara E., Rudkevich D. M., and Dasgupta P. K., Talanta, 77, 1814–20 (2009).
- Chapados C., Germain D., and Leblanc R. M., Can. J. Chem., 59, 2402–2411 (1981).
- Guidi L., Nali C., Ciompi S., Lorenzini G., and Soldatini G. F., J. Exp. Bot. , 48 , 173–179 (1997).
- Bevilaqua R. C. A., Zanella I., and Fagan S. B., Chem. Phys. Lett., 496, 310–315 (2010).
- Fanning J. C., Mandel F. S., Gray T. L., and Datta-Gupta N., Tetrahedron, 35, 1251–1255, (1979).
- Chapados C., Germain D., and Leblanc R. M., Biophys. Chem., 12, 189–198 (1980).
- Lin-Vien D., Colthup N. B., Fateley W. G., and Grassell J. G., The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules, Academic Press, San Diego, CA, (1991).
- Catalano M. M., Crossley M. J., Harding M. M., and King L. G., J. Chem. Soc., Chem. Commun., 1535–1536 (1984).

This work is licensed under a Creative Commons Attribution 4.0 International License.









