A Facile Microwave-Assisted Synthesis of Some Fused Pyrimidine Derivatives
S. A. Al-Issa
Chemistry Department, Faculty of Science, Princess Nora Bint Abdul Rahman University, Riyadh, Saudi Arabia.
DOI : http://dx.doi.org/10.13005/ojc/300209
Article Received on :
Article Accepted on :
Article Published : 16 Apr 2014
The highly accelerated synthesis of thienopyrimidinones, theino- pyrimidines,thioxotheinopyrimidinones and a thienotriazolopyrimidinone derivatives under microwave irradiation is reported. Compared to conventional conditions, microwaves method offered several advantage likes short time, good yields, simple procedure, mild conditions and easy workup. The structure of synthesized compounds have been characterized on the basis of their elemental analysis and spectral data, and screened for their antimicrobial activity.
KEYWORDS:Thienopyrimidine; Thioxothienopyrimidine; Triazolopyrimidinone; Microwave Irradiation
Download this article as:| Copy the following to cite this article: Al-Issa S. A. Facile Microwave-Assisted Synthesis of Some Fused Pyrimidine Derivatives. Orient J Chem 2014;30(2). |
| Copy the following to cite this URL: Al-Issa S. A. Facile Microwave-Assisted Synthesis of Some Fused Pyrimidine Derivatives. Orient J Chem 2014;30(2). Available from: http://www.orientjchem.org/?p=3050 |
INTRODUCTION
Pyrimidines and fused pyrimidines compounds, are a big family of the heterocyclic compounds, which have been focus of great interest because of their wide range of biological and pharmacological activities as antibacterial and antifungal activities(1-4), anticancer(5-8), anti-inflammatory(9) and many thieno[2,3-d] pyrimidine derivatives have been proved to use in case of Alzheimer and Parkinson disease(10).
Microwave irradiation has attracted attention of organic synthesis due to the reduction in reaction times, good yields and cleaner reaction than conventional procedure(11-16). There are several examples describing the advantage of using microwave irradiation for synthesis the thieno [2,3-d]pyridine-4(3H)ones for example , Hesse reported the synthesis of thieno[2,3-d]pyrimidin-4(3H)-ones under microwave irradiation in good yield(17).
It seemed most interesting to extend the scope of synthesize of pyrimidine derivatives having active moieties under microwave irradiation with the aim to evaluate their biological activity as antibacterial or antifungal agents.
EXPERIMENTAL
Melting point are uncorrected . Microwave reaction were performed on a microwave oven start SYNTH-Milestone-open vessel. IR spectra were recorded in potassium bromide on Perkin-Elmer 380 and 387 spectrometer. 1H-NMR spectra were run on a JEOL-JNMGX-300 and Junm Ex-400 in DMSO-d6 or CDCl3 using TMS as internal standard and chemical shift were expressed as part of million, ppm ( values) against TMS and internal references. Electron impact (E1) MS spectra were obtained with aid of a ShimaDZU Gemsqp 5050. A spectrometers at 70 ev. Elemental analysis were recorded on a Heruss CHN analyser.
General procedure for synthesis of 5,6-disubtituted thieno[2,3-d]pyrimidin-4(3H)-ones (4a-c)
A mixture of 1a,b ( 1 mmol) and formamide ( 5-6 mmol) in ( 1 mmol) acetic acid, was irradiation in the microwave oven for10 min. at (300 W) 130oC. The reaction mixture was cooled and poured into iced-cold water, filtered, washed and recrystallized from ethanol.
5,6-Dimethyl -3H-thieno[2,3-d]pyrimidin-4-one (4a): M.p. 180-183oC; IR(cm-1): 3320, 1670; 1H-NMR(DMSO-d6)
1.7(s, 3H, CH3), 2.0(s, 3H, CH3), 7.80 (1H, s, pyrimidine -H), 10.00 (br. s, 1H, NH); MS: m/z (%), 180[M+] (95) (C8H8N2OS);CHN anal. calcd %: C:53.33; H: 4.44; N:15.55; Found: C: 53.45; H: 4.30; N:15.56.
5,6,7,8-Tetrahydro-3H-benzo[4,5]thieno[2,3-d]pyrimidin-4one(4b)
M.p. 257-259oC, IR(cm-1): br. 3400, 1670; 1H-NMR (DMSO-d6): 1.8-1.87 (m, 4H, CH2 at C-6 and C-7), 2.65-2.70 (m, 2H, CH2 at C-5); 2.80-2.89 (m, 2H, CH2 at C-8), 7.80 (s, 1H, pyrimidine -H), 10.90 (br. s, 1H, NH); MS: m/z (%) 206 [M+](90) (C10H10N2OS); CHN Anal. Calcd . % : C, 58.25; H,4.85; N, 13.59 Found: C, 58.30; H, 4.83; N, 13.60.
3,5,6,7,8,9-Hexahydrocyclohepta[4,5]thieno[2,3-d]pyrimidin-4-one (4c)
M.p.: 212-215oC; IR (cm-1): br 3410, 1710; 1H-NMR(DMSO-d6): 1.56-1.58 (m, 4H, 2CH2 at C-6 d C-7); 1.83-189 (m, 2H, CH2 at C-8), 2.70-283 (m, 2H, CH2 at C-5), 2.96-3.0 (m, 2H, CH2 at C-9), 8.01 (s, 1H, pyrimidine -H) 12.5( br. s, 1H, NH); MS: m/z (%) 220[M+] (100) (C11H12N2OS); CHN . Anal. Calcd. %: C:60.00; H:5.45, N: 12.72; Found: C: 60.10, H: 5.47; N: 12.70.
4-Chloro-5,6,7,8-tetrahydrobenzo[4,5] thieno[2,3-d] pyrimidine (7): Conventional method
A mixture of 4b (5 mmol) and phosphorus oxychloride (15 ml) was heated under reflux for 20 h. After completion of reaction, The excess phosphorus oxychloride was removed by distillation under reduced pressure ,the residue left was titrated with sodium bicarbonate solution (10%); the solid separated was filtered, dried and recrystallized from toluene.
Microwave method
A mixture of 4b(5 mmol) and phosphorus oxychloride (15 ml) was irradiated in microwave oven at( 60 W) 120oC for 15 min.. After completion of reaction, The workup was carried out as described for conventional method. M.p: 93-96oC ; IR (cm-1): 1620, 1580; 1H-NMR(DMSO-d6): 1.90-1.94, (m, 4H, 2CH2, at C6 & C7), 2.90-2.94 (m, 2H, CH2 , C-5), 3.00-3.40 (m, 2H, CH2 at C-8), 8.72 (d, 1H pyrimidine -H); MS: m/z (%) 224[M+] (56%) ( C10H935Cl N2S), 226[M+2] (30)( C10 H937ClN2S);CHN: Anal. Calcd.: C: 53. 57: H: 4.01; N: 12.50.Found C: 53.21, H: 4.20, N: 12.49.
General procedure for synthesis of 4-N-(aryl)- 5,6,7,8-tetrahydrobenzo [4,5]thieno[2,3-d]pyrimidines (8):
conventional method:
A solution of compound 7 (10 mmol) and aryl amine (2.0 mmol) in 2-propanol (30 mL) was stirred under reflux for 12h., the solvent was removed under reduced pressure and the solid product was filtered dried and washed several times with petroleum ether.
Microwave Method
A mixture of compound 7 (1.0 mmol) and aryl amine (2.0 mmol) in 2-propanol (10 mL) was stirred for 5 min., then the mixture was irradiated in the microwave oven at (60W) 100oC for 20 min. The workup was carried out as described for conventional method.
4-N-(4-methylphenyl)-5,6,7,8-tetrahydro[4,5]thieno[2,3-d] pyrimidine (8a)
M.p . oC : 235-237oC; IR (cm-1): br. 3410; 1620; 1H-NMR(DMSO-d6): 1.85-1.98 (m, 4H, CH2 at C-6, C-7) 2.28(s, 3H, CH3), 2.34-2.64 (m, 2H, CH2 at C-5) 2.9-3.04 (m, 2H, CH2 at C-8), 7.1-7.80 (m,4H,Ar-H) , 7.95-8.1, ( br.s, 1H, NH), 8.22 (s, 1H, pyrimidin-H). MS: m/z (%) 295[M+] (95%).( C17H17N3S); CHN: Anal. Calcd .:C:69.15.H: 5.76; N: 14.23. Found: C: 69.30, H: 5.31; N: 14.34.
4-N-(3-Trifluoromethylphenyl)-5,6,7,8- tetrahydrobenzo[4,5]thieno [2,3-d] pyrimidine (8b)
M.poC. 224-225 oC; IR (cm-1): br. 3390, 1610; 1H-NMR (DMSO-d6): 1.80-1.87(m, 4H, 2CH2 at C-6 d C-7), 2.40-2.60(m, 2H, CH2 at C-5), 2.93-3.00 (m, 2H, CH2, at C-8), 7.31 -7.80 (m, 4H, Ar-H), 8.10(s, 1H, pyrimidin-H) ,8.7-8.9 (br. s, 1H, NH); MS: m/z (%) 349(100) ( C17H14F3N3S); CHN : Anal. Calcd. (%) C: 58.45; H: 4.01; N: 12.03; Found: C, 58.36; H: 4.36; N: 12.98.
4-N-(2,5-Dimethylphenyl)-5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d] pyrimidine(8c)
M.poC. 213-215oC; IR (cm-1): br. 3140, 1610; 1H-NMR (DMSO-d6): 1.80-1.87 (m, 4H, 2CH2 at C-6d C-7), 2.31(s, 3H, CH3), 2.40 (s, 3H, CH3), 2.44 (m, 2H, CH2 at C-5), 2.94-2.97 (m, 2H, CH2 at C-8), 7.16-7.80 (m, 3H, Ar-H), 8.18 (s, 1H, pyrimidine -H) ,8.41( br. s, 1H, NH); MS: m/z (%); 309 (60%). C18H19N3S; CHN :Anal. Calcd. (%): C, 69.90, H: 6.14, N: 13.59; Found: C: 69.93; H 69.92.
General procedure for synthesis of 3,5,6-trisubtituted-1H-2-thioxo thieno[2,3,-d] pyrimidin-4-ones(11)
A mixture of 1b ( 1 mmol) and the appropriate isothiocyanate (1.1 mmol) in (10 ml) acetic acid, the mixture was irradiated in microwave oven at (300 W)120oC for 20 min. The cold reaction was poured onto crushed ice, the solid obtained was filtered, washed with water, dried and recrystallized from ethanol.
3-Allyl-2-thioxo-2,3,5,6,7,8-hexahydro-1H-benzo[4,5]thieno[2,3-d] pyrimidin-4-one(11a)
M.p.: 234-237oC, IR (cm-1) 3330, 1710, 1210; 1H-NMR (DMSO-d6): 1.68-1.73, (m, 4H, 2CH2 at C-6 d C-7), 2.30-2.50(m, 2H, CH2 at C-5), 2.89-2.90(m, 2H, CH2 at C-8); 4.35-4.50(br. m, 2H, NCH2), 5.41-5.60 (m, 2H, =CH2); 5.89-6.30 (m, 1H, HC=), 11.9(s, 1H, NH); MS: m/z (%); 278(100) ( C13H14N2OS2 ); CHN: Anal. Calcd.: C, 56.11; H: 5.03; N: 10.07; Found: C: 56.60; H: 4.34; N: 9.85.
3-(Phenyl)-2-thioxo-5,6,7,8-tetrahydro-1H-benzo[4,5]thieno[2,3-d] pyrimidin-4-one(11b)
M.p.:261-263oC;IR(cm-1):3400,1700,1200;1H-NMR(CDCl3): 1.80-1.84 (m, 4H, 2CH2 at C-6 d C-7), 2.58-2.61 (m, 2H, CH2 at C-5), 2.85-2.90 (m, 2H, CH at C-8), 7.30-7.60 (m, 5H, Ar-H), 11.5(br.s, 1H, NH) MS: m/z (%) 314[M+](100) C16H14N2OS2; CHN :Anal. Calcd, C: 61.14; H: 4.45; N: 8.91, Found: C: 61.20; H:4.53; N: 8.90.
3-(4-Chlorophenyl)-2-thioxo-5,6,7,8-tetrahydro-1H-benzo[4,5]thieno [2,3-d]pyrimidine-4-one (11c)
M.p.: 289-291oC, IR (cm-1) 3200, 1700, 1220; 1H-NMR(CDCl3): 1.70-1.76 (m, 4H, CH2 at C6 & C7) 2.60-2.64 (m, 2H, CH2 at C-5); 2.71-2.74 (m, 2H, CH2 at C8), 7.30-7.50 (m, 4H, Ar -H); 8.5(br.s, 1H, NH); MS: m/z (%) 348[M+] (90) C16H13Cl35N2OS, 350[M+2] (25)( C16H13Cl37N2OS2); CHN: Anal. Calcd (%): C, 55.09; H,3.73; N, 8.03; Found: C, 55.30; H, 3.76; N, 8.09.
General procedure for synthesis of : 3-Substituted-2-hydrazino-5,6,7,8-tetrahydro-3H-benzo[4,5]thieno[2,3-d]pyrimidin-4-ones.(12).
A mixture of compound 11b (1.0 mmol), and hydrazine hydrate (99%)(2.0 mmol) in ethanol (15 ml) was submitted to microwave irradiation for 5 min. at( 60W) 100oC. After cooling the reaction mixture poured into ice cooled water and stirred for 10 min the solid product was filtered, dried and recrystallized from ethanol.
3-Allyl-2-hydrazino-5,6,7,8-tetrahydro-3H-benzo[4,5]thieno[2,3-d] pyrimidin-4-one(12a)
M.p.196-199oC; IR (cm-1): 3390, 3310,, 1610, 1580, 1H-NMR: 1.70-1.73 (m, 4H, 2CH2 at C-6 & C-7); 2.61-2.68(m, 2H,, CH2 at C-5); 2.80-2.82(m, 2H, CH2 at C-8), 4.01-4.30 (br. s, 2H, NH2), 4.50-4.56(m, 2H, NCH2), 4.96(d d, 1H, J =10.5 Hz, =CH2), 5.12, (dd, 1H, J= 17.5 Hz, =CH2), 5.80-5.84(m, 1H, CH=); 8.5 (br. s, 1H, NH). MS: m/z(%) 276 [M+] (90%),( C13H16N4OS); CHN Anal. Calcd.%: C, 56.52; H, 5. 79; N, 20.28; Found :C,56.56; H, 5.83; N,20.24.
3-(4-Chlorophenyl)-2-hydrazino-5,6,7,8-tetrahydro-3H-benzo[4,5]-thieno[2,3-d]pyrimidin-4-one(12b)
M.p.204-206oC; IR(cm-1), 3410 (br.),1610, 1580 ; 1H-NMR (DMSO-d6): 1.74-1.80(m, 4H, 2CH2 at C-6 d C-7); 2.63-2.70( m, 2H, CH2 at C-5), 2.80-2.83 (m, 2H, CH2 at C-8), 4.3 (br. s, 2H, NH2), 7.4-7.9 (m, 4H, Ar-H), 8.01 (br. s, 1H, NH); MS: m/z(%) 346 [M+] (90) (C16 H15 35Cl N4OS), 348[ M+2] (25) (C16H1537ClH4OS); CHN : Anal. Calcd: C,55.49; H, 4.33; N , 16.18 ; Found: C, 55.45; H: 4.30; N: 16.14.
4-Allyl-6,7,8,9-tetrahydro-4H-benzo[4,5]thieno[2,3-d][1,2,4]triazolo [3,4,-b]pyrimidin-5-one (13a)
A mixture of compound 12a(1.0 mmol) and formic acid (10 ml) was submitted to microwave irradiation for 10 min. at (60 W)800C after cooling the reaction mixture was poured into ice-cooled water, the formed solid was collected by filtration washed several time with ethanol, dried and recrystallized from ethanol.: M.p. 213-215oC, IR (cm-1), 1680, 1610, 1H-NMR (CDCl3): 1.80-1.86 (m, 4H, 2CH2 at C7 and C-8), 2.60-2.78( m, CH2 at C-6), 3.00-3.42 (m, 2H, CH2 at C-9); 4.50-4.56(m, 2H, NCH2), 4.96(dd, 1H, J =10.5 Hz, =CH2), 5.12, (dd, 1H, J= 17.5 Hz, =CH2), 5.80-5.84(m, 1H, C-H=); 8.5 (br. s, 1H, NH). 9.30 (s, 1H, triazolo -H); MS, m/z = 286 (90) (C14H14N4OS); CHN : Anal. Calcd :C, 58.74 ;H, 4.89; N: 19.58. Found: C: 58.79; H: 4.80; N; 19.55.
General procedure for synthesis of: 4-Substituted-1-methyl-6,7,8,9-tetrahydro-4H-benzo[4,5]thieno[2,3-d][1,2,4]triazolo[3,4-b] Pyrimidine 5-ones(13b,c).
A mixture of compound 12a,b (1.0 mmol) and acetic acid (10 ml) was submitted to microwave irradiation for 10 min at ( 60W )80oC. After cooling the reaction mixture was poured into ice-cooled water. The solid product was collected by filtration washed several time with ethanol, dried and recrystallized from ethanol.
4-Allyl-1-methyl-6,7,8,9-tetrahydro-4H-benzo[4,5]thieno[2,3-d][1,2,4] triazolo [3,4-b] pyrimidin-5-one (13b).
M.p.: 228-230oC; IR (cm-1):1700, 1610; 1H-NMR (DMSO-d6): 1.78-1.89 (m, 4H, 2CH2 at C-7& C-8), 2.6 (m, 2H, CH2 at C-6) ,2.76 (s, 3H, CH3),2.96-3.2 (m, 2H at C-9); 4.57-4.70 (m, 2H, N-CH2), 5.10 (dd, 1H, J= 19.8, CH2=), 5.40 (dd, H, J = 17.5, CH2=‘) 6.03(m, 1H, HC=); MS: m/z [M+] 300 (100); C15H16N4OS. CHN : Anal. Calcd: C,60.00; H, 5.33; N, 18.66, Found: C,60.50; H, 5.40; N,18.65.
4-(4-Chlorophenyl)-1-methyl-6,7,8,9-tetrahydro-4H-benzo[4,5] thieno[2,3-d][1,2,4] triazolo[3, 4,-b] pyrimidin-5 -one (13c)
M.p.: 300<, IR (cm-1): 1680, 1610; 1H-NMR (DMSO-d6): 1.80-1.86 (m, 4H, 2CH2 at C-7 d C-8) 2.5 -268(m, 2H, CH2 at C-6); 2.73 (s, 3H, CH3), 3.00-3.20 (m, 2H, CH2 at C-9) 7.30-7.70 (m, 4H, Ar-H); MS: m/z(%) 370 [M+] (100) (C18H1535ClN4OS; 372[M+2] (40)(C18H1537ClN4OS); CHN: Anal. Calcd. C: 58.29; H: 4.04; N: 15.11. Found :C, 58.62; H, 4.09; N, 15.12.
General procedure for synthesis of: 4-Substituted-1-mercapto-6,7,8,9-tetrahydro-4H-benzo[4,5]thieno[2,3-d][1,2,4]triazolo[3,4-b]pyrimidin-5-ones (14a,b).
A mixture of 12a,b (1.0 mmol) and carbon disulphide (10 mL) in pyridine (10 mL) was stirred for 10 min., then submitted to microwave irradiation for 10 min at (60 W) 40oC. The mixture was evaporated under reduced pressure and the residue was treated with ethanol. The formed solid was filtered and washed several times with water, dried, and recrystallized from ethanol.
4-Allyl-1-mercapto-6,7,8,9- tetrahydrobenzo[b] thieno[2,3-d][1,2,4] triazolo[3,4-b] pyrimidin 5(4H) one (14a)
M.p. : 246-248 oC, IR (Cm-1): 3200, 1680, 1210; 1H-NMR (CDCl3-d6) 1.78-1.81(m, 4H, CH2 at C-7 d C-8), 2.65-2.71 (m, 2H, CH2 at C-6); 2.95-3.00 (m, 2H, CH2 at C-9); 4.48-4.60 (m, 2H, N-CH2), 5.00 (dd, 1H, J= 9.8 Hz, =CH2); 5.27 (dd, 1H, J= 16.8 Hz, =CH2) 5.86-5.91 (m, 1H, HC=); 10.6 (br. s, 1H, SH); MS: m/z(%) ,[M+] 318 (100) (C14H14N4OS2); CHN: Anal. Cacld. (%): C, 52.83; H , 4.40; N, 17.61. Found C: 52.89; H: 4.48; N: 17.69.
4-(Chlorophenyl)-1-mercapto-6,7,8,9-tetrahydrobenzo[b]thieno[2,3-b] [1,2,4] triazolo[3,4-b] pyrimidin-5 (4H)-one (14b)
M.p.: 260-263 oC, IR (cm-1): 3300, 1600, 1610, 1200; 1H-NMR (CDCl3-d3): 1.80-1.84 (m, 4H, 2CH2 at C-7 d C-8); 2.50-2.62 (m, 2H, CH2 at C-6); 2.95-3.2(m, 2H, CH2 at C-9), 7.1-7.80 (m, 4H, Ar-H), 11.5 (br. s, 1H, SH); MS: m/z(%) ,[M+] 388 (90) (C17H1335ClN4OS2), 390[M+2] (25) (C17H1337ClN4OS2); CHN Anal. Calcd. (%) : C, 52.50; H , 3.34; N, 14.41. Found: C, 52.59; H, 3.40; N, 14.40.
Results and Discussion
The starting materials, namely 2-amino-3-(carboxyamide or carboxylic acid)-4,5-disubtituted thiophene 1a,b were prepared according to the corresponding literature methods(18,19).
The reaction of 1a,b and formamide under a microwave irradiation with reactants ratio of 1:2 at 1:5-6 in the presence of one equivalent of acetic acid at (300 W) for 10 min 130oC yielded thienopyrimidinone derivatives 4a-c (Scheme 1, Table 1).
Table 1: Microwave experimental condition for the synthesis of compound 4
|
Compound No. |
R1 |
R2 |
X |
Substrate ratio |
Temp. (0C) |
Power in put (W) |
Yield (%) |
M.p. (0C) |
Lit..m.p21 (0C) |
|
1a |
CH3 |
CH3 |
NH2 |
1:6 |
130 |
300 |
85 |
180-183 |
|
|
1b |
(CH2)4 |
NH2 |
1:5 |
130 |
300 |
86 |
257-259 |
255-275 |
|
|
1c |
(CH2)5 |
NH2 |
1:5 |
130 |
300 |
81 |
210-211 |
209-211 |
|
|
1a |
CH3 |
CH3 |
OH |
1:6 |
130 |
300 |
79 |
180-183 |
— |
|
1b |
(CH2)4 |
OH |
1:5 |
130 |
300 |
83 |
257-259 |
255-275 |
|
|
1c |
(CH2)5 |
OH |
1:5 |
130 |
300 |
81 |
210-211 |
209-211 |
The next step we converted 3H-thieno[2,3-d]pyrimidin-4-one 4b in to 4-chlorothieno[2,3-d]pyrimidine derivatives 7 by the action of phosphorous oxychloride using both classical and microwave reaction(11,12,20-22); the latter method afforded higher yields of the required products in shorter time.(Scheme 3, Table 2)
Table 2: Experimental conditions used for the microwave assisted and classical methods synthesis of compounds 7 & 8.
|
Compound. No. |
Ar |
Microwave methoda |
Classical Methodb |
M.p. (0C) |
Lit..m.p21 (0C) |
||
|
Reaction time (min.) |
Yield % |
Reaction time (h.) |
Yield % |
||||
|
7a |
— |
15 |
75 |
20 |
48 |
93-95 | 90-92 |
|
8a |
4-CH3-C6H4 |
20 |
85 |
12 |
13 |
235-237 | — |
|
8b |
3-CF3C6H4 |
20 |
76 |
12 |
12 |
224-225 | 224-226 |
|
8c |
2,5-CH3C6H4 |
20 |
84 |
12 |
15 |
213-215 | — |
a; Reaction conditions: 2-PrOH, reflux under MW irradiation (60 W)
b: Reaction conditions: 2-PrOH, reflux temperature.
Substitution with an aromatic amine was performed on chloro derivatives under classical and microwave irradiation to produce the anilino derivatives8a-c. (Scheme 3, Table 2) .The structure of synthesized compounds were assigned on the basis of its spectral data and elemental analysis.
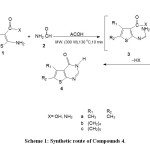 |
Scheme 1: Synthetic route of Compounds 4. Click here to View Scheme |
The reaction mechanism may proceed via an o-amidine intermediated 3 as illustrated in (Scheme 2)11:
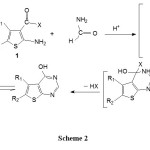 |
Scheme 2 |
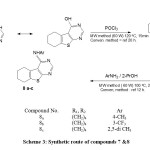 |
Scheme 3: Synthetic route of compounds 7 &8 |
Next attention was focused on the prep. In the classical synthesis of this compounds, a mixture of starting material reacted with alkyl or aryl thio isocyanates in ethanol. This method provided low to moderate yield and long reaction time(22). We have recently synthesis the target compounds 11a-c using microwave irradiation in acetic acid (Scheme 4, Table 3).
Table 3: Microwave experimental conditions for the synthesis of compounds 11-14
|
Comp. No. |
R |
Temp. (oC) |
Power in put (W) |
Time (min.) |
Yield (%) |
M.P. (oC) |
Lit.m.p21 (oC) |
|
11a |
CH2CH=CH2 |
120 |
300 |
20 |
85 |
234-235 | —— |
|
11b |
C6H5 |
120 |
300 |
20 |
87 |
261-263 | 259-261 |
|
11c |
4-ClC6H4 |
120 |
300 |
20 |
85 |
289-291 | 289-290 |
|
12a |
CH2CH=CH2 |
100 |
60 |
5 |
80 |
196-198 | —— |
|
12b |
4-ClC6H4 |
100 |
60 |
5 |
86 |
204-206 | 204-204 |
|
13a |
CH2CH=CH2 |
80 |
60 |
10 |
87 |
213-215 | —— |
|
13b |
CH2CH=CH2 |
80 |
60 |
10 |
83 |
228-230 | —— |
|
13c |
4-ClC6H4 |
80 |
60 |
10 |
85 |
>300 | 300 |
|
14a |
CH2CH=CH2 |
40 |
60 |
10 |
83 |
246-248 | —— |
|
14b |
4-ClC6H4 |
40 |
60 |
10 |
87 |
260-263 | —— |
The MW method requires higher yields, short reaction time, and eco friendly. Reaction of 11a-cwith hydrazine hydrate under microwave irradiation at 60 W(100oC) for 5 min., afforded 2-hydrazino derivatives 12a,b.(Scheme 4, Table 3).
Cyclization of compound 12a,b by aliphatic carboxylic acid, namely Formic or acetic acid under MW irradiation for 10 min at (60W) 80oC gave the triazolo derivatives 13aand 13b,crespectively (Scheme 4,Table 3).
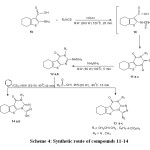 |
Scheme 4: Synthetic route of compounds 11-14 |
Finally, treatment of 12a,b with carbon disulphide under microwave irradiation for 10 min. yielded mercaptotriazolo derivatives 14a,b(Scheme 4, Table 3).
The structure of compounds 11-14 were confirmed from their spectral data and elemental analysis.
ANTIMICROBIAL ACTIVITY:
The antimicrobial activity of the compounds considered was tested on the following organisms:
- Candida albicans ATCC No. 10231.
- Staphylocoecus Aureus ATCC No. 6538.
- Eschierichia Coli ATCC No. 8739.
- Proteus mirabilis ATCC No. 29906.
The biological activity was planned according to the cup-plate method adopted with some modify (23).
What man No. 2 filter paper disk (6.5 cm) was impregnated with 200 mg of the compound. The disk was placed on the surface of the cold solid medium petridishes vaccinated with considered organisms and then incubated at 5oC for 1h. to permit good diffusion and then transferred to an incubated at 28oC for 24 h.
A summary of the biological activity results is shown in Table 4.
Table 4: Antimicrobial activity of the compounds against bacteria and fungi.
|
Test |
Compounds |
|||||||
|
Organism |
5 |
7 |
8 b |
11a |
12b |
13a |
13b |
14a |
|
1 |
+ |
+ |
– |
+ |
– |
– |
+ |
– |
|
2 |
+ |
++ |
+ |
– |
– |
– |
+ |
+ |
|
3 |
+ |
+ |
– |
– |
– |
– |
+ |
+ |
|
4 |
– |
– |
– |
+ |
+ |
– |
– |
– |
The sensitivity of microorganisms to the compound is identified in the following manner:
+++ = highly sensitive (inhibition zone 1.2-1.5 mm)
++ = fairly sensitive (inhibition zone 1.2-0.9 mm)
+ = Slightly sensitive (inhibition zone 0.9-0.6 mm)
– = not sensitive.
References
- Litvinov V. P.; Russian Chemical Bulletin, 53(3) , 487(2004).
- Alagarsmy V., Murugarathan G. Venkateshperumal R., Biol. Pharm. Bull, 26, 1711(2003).
- Keshav C. P., International Journal of Chem. Tech. research; 5(1), 141(2013).
- Wang Y. D., Johnson S., Power A., Ginnis MC.; Miranda M. and Robindrah S. k., Bioorg. Med. Chem. Lett. , 15, 2763(2005).
- Baselga J., Averbuch, S. D., Drugs, 60, 33(2000).
- Nargues S., Soliman R., El-Tombary A. A., El-Hawash S. A., Shaaban O. G.; Medical Chem. Research, 22(7), 3289 (2013)
- L. Dang, H. Gui, Q. Zhan, Y. Hu, S. Gni ,Wuhan University J. of Natural Science, 17(21)77( 2012).
- Elazab A.A, Al-Omar M. A., Abdel-Aziz A. A. M., Abdel-Aziz N. I., El-Sayed M. A., Aleisa A. M., Sayed-Ahmed M. , Abdel-Hamide S. G., Eur. J.Med. Chem., 45, 4188 (2010).
- Vena K., Sharvan L. N., Shachindra L. N.,. Narendrath Chandra J. N., and Nurgund L. V.; Der pharma Chemica, 4(2), 581(2012).
- Chaykovsky M., Lin M., Rosowsky A. and Modest E.; J. Med. Chem., 16 , 188(1977).
- Fenj L., Yiqing F., Quingging M., Wenchua L., Zhiming L., Quanrui W., Fenggang T., Arkiovoc (i), 40 ( 2007)
- Besson, T. and Chosson, E.; Comb. Chemis. High Throughput Screen. ,10, 1903(2007).
- Akhil A. N., Ashish p., Rajesh K. S., Kishore R. D. , Pathi L. G; Pharmagene, 1, 1(2013).
- Sarika M., Neelam S., Madhuri V., Jitendra V., Pinki B. P. , Suresh C. A.; Bull. Korean Chem. Soc.; 28(12), 2338(2007).
- Mizuno T., Iwai, T. , Ishino Y., Tetrahydron Lett.,45, 7073(2004).
- Alexandre, F. R., Berecobar, A., Besson, T.; Tetrahydron Lett., 43, 3911(2002).
- Hesse S. Perspicace E. , Kirch G., Tetrahydron Lett., 48, , 5261(2007).
- Sabins R. W.; Rangnekar, D. W.; Sonawane N. D., J. Heterocyclic Chem. 36, 333(1999).
- Gewald K. and Schinike E. Chem. Ber.; 99,94(1966).
- Liu G., Song B., Yang S., Yue W. Hu D. , Jin L and Lu S., Molecules ,11, 272(2006).
- El-Baih F. E., Al-Blowy H. A. S. , Al-Hazimi M., Molecules, 11, 498(2006).
- El-Hiti G. A., Hussain A., Hegazy A. S. , Alotaibi M. H., J. of Sulfur Chemistry 32(4), 361(2011).
- Abau-Zeid A. A. and Shehata Y. M., Indian J. Pharmacy, 31, 72 (1969).

This work is licensed under a Creative Commons Attribution 4.0 International License.









