Reaction of 2-R 5-Oxo 5-H 6- Ethylcarboxylate 7-Phenyl-[1,3,4]Thiadiazolo-[3,2-A]Pyrimidine with Morpholin and their Properties
Reza Moradivalikboni 1,* Zabialah Heidarnezhad2, Fatemeh Heidarnezhad2 , Yuldashboy Hozhiboev1 and Rahman Rahmanov1
1V.I.Nikitin Institute of Chemistry Academy of Sciences of the Republic of Tajikistan
2Department of Chemistry,Andimeshk Branch, Islamic Azad University, Andimeshk , Iran
DOI : http://dx.doi.org/10.13005/ojc/300156
Article Received on :
Article Accepted on :
Article Published : 03 Apr 2014
this article presents Synthesis of 2-R5-oxo 5-H 6 -Carbomorpholin 7-phenyl 1,3,4-thiadiazolo-[3,2-a] pyrimidine through reaction of 2- R 5 - Oxo 5 - H 6- EthylCarboxilate 7 – phenyl -1, 3,4 – Thiadiazolo-[3,2-a] pyrimidine with morpholin. in particular,for the new antibacterial drugs in these homologousseries of compounds, we have synthesized 2-R5-oxo 5-H 6 -Carbomorpholin 7-phenyl 1,3,4-thiadiazolo-[3,2-a] pyrimidine .The structures of the compounds obtained are set NMR, 13C, IR- spectroscopy.
KEYWORDS:2-R 5-oxo 5-H 6 -Carbomorpholin 7-phenyl 1;3;4-thiadiazolo-[3,2-a] pyrimidine - 2- R 5-Oxo 5-H 6-EthylCarboxilate 7 – phenyl -1, 3,4 –Thiadiazolo-[3,2-a] pyrimidine –Morpholin -Synthesis - The reaction.
Download this article as:| Copy the following to cite this article: Moradivalikboni R,* Heidarnezhad Z, Heidarnezhad F, Hozhiboev Y and Rahmanov R. Reaction of 2-R 5-Oxo 5-H 6- Ethylcarboxylate 7-Phenyl-[1,3,4]Thiadiazolo-[3,2-A]Pyrimidine with Morpholin and their Properties. Orient J Chem 2014;30(1). |
| Copy the following to cite this URL: Moradivalikboni R,* Heidarnezhad Z, Heidarnezhad F, Hozhiboev Y and Rahmanov R. Reaction of 2-R 5-Oxo 5-H 6- Ethylcarboxylate 7-Phenyl-[1,3,4]Thiadiazolo-[3,2-A]Pyrimidine with Morpholin and their Properties. Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=2815 |
The diverse and interesting biological activity of thiadiazoleshas been reported 1-4It is well known that these heterocyclesare valuable building blocks. Many methods for preparationof these heterocyclic ring systems and their fused analogues have been described in the literature 5-6. 1,3,4-thiadiazoles provided a usefulmethod for the synthesis of thiadiazolopyrimidine 7. Pyrimidine derivatives have been found to be associated with diverse biological activities and numerous reportshave appeared in the literature 8-12. This highlighted their chemistry and use. The pyrimidine derivatives have remarkable pharmacological activity 13,14 and widely used in the field of anti-microbial, antiviral, etc. Thiadiazole derivatives were shown to possess many biological activities including anti-inflammatory 15-16.The The introduction of a substituent at position 6 of the1,3,4-thiadiazolo [3,2-a] pyrimidine system efficientlyenhances the physiological activity of the molecule17-19. This replacement occurs in the reactions of 1,3,4 -thiadiazolo [3,2-a] pyrimidine derivatives withelectrophiles20,21.Derivatives of 1,3,4-thiadiazolo [3,2-alpyrimidine are potential biologically active substances,22-25 The introductionof ketene dithioacetal fragments into the moleculesmakes it possible to synthesize heterocyclic systemswith various functional groups26,27. We Preparated2-R5-oxo 5-H 6 -Carbomorpholin 7-phenyl 1,3,4-thiadiazolo-[3,2-a] pyrimidine in two stage. In step first we have synthesize2-R5-oxo5-H6-EthylCarboxilate7-phenyl 1,3,4-thiadiazolo[3,2,-a]pyrimidine(3) with use2- R 5-amino 1,3,4- thiadiazole(1) andethyl 2- formyl 3- oxo 3- phenyl propanoate (2 )(Figure 1).
![Table 1.synthesisof 2- R 5-oxo 5-H 6-Carbomorpholin 7-phenyl -1,3,4-thiadiazolo [3,2-a] pyrimidinefrom 2- R 5-oxo 5-H 6-ethylcarboxylate 7-phenyl 1 ,3,4- thiadiazolo [3,2-a] pyrimidine and morpholina](http://www.orientjchem.org/wp-content/uploads/2014/04/Vol30_No1_React_Reza_fig1-150x150.jpg) |
Figure 1.synthesisderivatives of 2-R5-oxo 5-H 6-ethylcarboxylate7-phenyl 1,3,4-thiadiazolo [3,2- a]pyrimidine |
In another stage 2-R5-Oxo5-H6-EthylCarboxilate7-phenyl 1,3,4-thiadiazolo-[3,2,-a]pyrimidinereacted with morpholin(4) until produced 2-R 5-oxo 5-H 6 –Carbomorpholin 7 -phenyl 1,3,4-thiadiazolo-[3,2-a] pyrimidine(5-9)(f2).
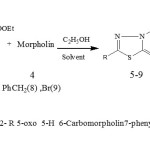 |
Figure 2. synthes is of 2- R 5-oxo 5-H 6-Carbomorpholin7-phenyl -1,3,4-thiadiazolo [3,2-a] pyrimidine Click here to View Figure |
Result and Discussion
we tried synthesisof 2- R 5-oxo 5-H 6-Carbomorpholin 7-phenyl -1,3,4-thiadiazolo [3,2-a] pyrimidinewith 2-R 5-oxo 5-H 6-ethylcarboxylate 7-phenyl 1 ,3,4-thiadiazolo [3,2-a] pyrimidine and morpholin in varioussolvent. But alcohols are the best solvents to this reaction .The alcoholssuch asmethanolandethanolalcoholhave more use. The herbicidal activities of the target compounds were evaluatedagainst a variety of weeds by flat-utensil method according with the standard bioactivity test.Applicability of this procedures, that we synthesis a wide variety of 2-R 5-oxo 5-H 6-R–amide derivatives7-phenyl 1 ,3,4- thiadiazolo [3,2-a] pyrimidine from 2-R 5-oxo 5-H 6- ethyl carboxylate7-phenyl 1,3,4-thiadiazolo [3,2- a]pyrimidine and morpholinin the presence of alcohol ethanol at 78 oC and obtained the desirable products in good to excellent yields (Table 1).
![Table 1.synthesisof 2- R 5-oxo 5-H 6-Carbomorpholin 7-phenyl -1,3,4-thiadiazolo [3,2-a] pyrimidinefrom 2- R 5-oxo 5-H 6-ethylcarboxylate 7-phenyl 1 ,3,4- thiadiazolo [3,2-a] pyrimidine and morpholina](http://www.orientjchem.org/wp-content/uploads/2014/04/Vol30_No1_React_Reza_T1-150x150.jpg) |
Table 1. synthesisof 2- R 5-oxo 5-H 6-Carbomorpholin 7-phenyl -1,3,4-thiadiazolo [3,2-a] pyrimidinefrom 2- R 5-oxo 5-H 6-ethylcarboxylate 7-phenyl 1 ,3,4- thiadiazolo [3,2-a] pyrimidine and morpholina Click here to View table |
Experimental
A mixture of 2-CH3 5-oxo 5-H 6-ethylcarboxylate 7-phenyl 1,3,4- thiadiazolo [3,2-a] pyrimidine (1 mmol),amin derivatives(1 mmol) was stirred magnetically at 78oC and the progress of the reaction was monitored by thin-layer chromatography (TLC). The reaction mixture was filtered.In all the cases, the product obtained after the usual work up gave satisfactory spectral data. For example,2-CH3 5-oxo 5-H 6-ethylcarboxylate 7-phenyl 1,3,4- thiadiazolo [3,2-a] pyrimidine (1 mmol-0.315gr),morpholin(1 mmol- 0.087gr)reacted to gether in alcoholethanol at 78 oC.Andtheproduct( 2-CH3 5-oxo 5-H 6-carbomorpholin 7-phenyl 1,3,4- thiadiazolo [3,2-a] pyrimidine )isobtainedin 85%yield. 2-CH3 5-oxo 5-H 6-carbomorpholin 7-phenyl -1,3,4-thiadiazolo [3,2-a] pyrimidine:1H NMR (400 MHz, CDCl3, δ ppm): 0.9(s,3H,CH3 ););3.65(t,2H,CH2);7.30-7.46 (5H, Ph); – 13C NMR (100 MHz, CDCl3, δ ppm): 24.2(CH3),45.5 (CH2),45.5 (CH2),66.2 (CH2), 66.2 (CH2), 118 (C), 126,4 (CH) , 126,4 (CH) ,128(CH), 128.7(CH), 128.7(CH), 136.9(C), 154.7(C), 159 .8(C),162.1(C), 163 (C),168(C).
Conclusions
Compound 2- R 5-oxo 5-H 6-Carbomorpholin 7-phenyl -1,3,4-thiadiazolo [3,2-a] pyrimidine were procedure in excellent yields from 2- R 5-oxo 5-H 6-ethylcarboxylate 7-phenyl 1,3,4- thiadiazolo [3,2-a] pyrimidine and morpholinthat have a broadspectrum of antimicrobial activity . The pyrimidine derivatives haveremarkable pharmacological activity and widelyused in the field of anti-microbial, antiviral. Such medicinal utilities of the Pyrimidine derivatives prompted to synthesize the new pyrimidinethiosemicarbazide,1,3,4-thiadiazole compounds.
References
- Carraro F, Pucci A, Naldini A, Schenone S, Bruno O, Ranise A,et al. Pyrazolo[3,4-d]pyrimidines endowed with antiproliferativeactivity on ductal infiltrating carcinoma cells. J Med Chem2004;47:1595–8.
- Mylari BL, Oates PJ, Zembrowski WJ, Beebe DA, Conn EL,Coutcher JB, et al. A sorbitol dehydrogenase inhibitor ofexceptional in vivo potency with a long duration of action: 1-(R)-{4-[4-(4,6-dimethyl[1,3,5]triazin-2-yl)-2R,6Sdimethylpiperazin-1-yl]pyrimidin-2-yl}ethanol. J Med Chem 2002;45:4398–401.
- Prekupec S, Makuc D, Plavec J, Suman L, Kralj M, Pavelic K,et al. Novel C-6 fluorinated acyclic side chain pyrimidinederivatives: synthesis, 1H and 13C NMR conformationalstudies, and antiviral and cytostatic evaluations. J Med Chem 2007;50:3037–45.
- Gazivoda T, Sokcevic M, Kralj M, Suman L, Pavelic K, DeClercq E, et al. Synthesis and antiviral and cytostatic evaluationsof the new C-5 substituted pyrimidine and furo[2,3-d]pyrimidine40,50-didehydro-l-ascorbic acid derivatives. J Med Chem2007;50:4105–12.
- Singh H, Yadav LDS, Shukla KN, Diwedi R. Ringtransformation of michael adducts of benzylidene-5-oxazolones and 2-amino-1,3,4-thiadiazoles to antifungal 6,7-dihydro-5(H)-thiadiazolo[3,2-a]pyrimidin-5-ones. J Agric Food Chem 1990;38:1962–4.
- Kornis G, Marks PJ, Chidester CG. Reaction of beta-oxoesterswith 2-amino-1,3,4-thiadiazoles. A reinvestigation. J Org Chem1980;45:4860–3.
- Cressier D, Prouillac C, Hernandez P, Amourette C, Diserbo M,Lion C, et al. Synthesis, antioxidant properties andradioprotective effects of new benzothiazoles and thiadiazoles.Bioorg Med Chem 2009;17:5275–84.
- Kape, C,Oliver 100 years of the BiginelliDihydropyrimidine synthesis, Tetrahedron, 1993, 49, 6937 – 6963.
- Ugi, 1.;Domling, A.; Horl, w. multi component Reactions in organic hemistry. Endeavour, 1998, 18, 115-122.
- Kape, C O, Eur J MedChem, 2000, 35, 1043-1055.
- C Mannich, & D Lammering, ChemBer, 1922, 5, 3510-3516.
- A Manjula, BV Rao& P Neelakantam, Synth Commun, 2004, 34, 2665-2672.
- J Andrew, Zych, Hong-Jun Wang, A Samuel, Sakwa, Tetrahedron Letters, 2010, 51, 5103-5105.
- Garima, P Vishnu, Srivastava, S LalDhar, Yadav, Tetrahedron Letters, 2010, 51, 6436-6438.
- V Sergey, Ryabukhin, S Andrey, Plaskon, S Semen, Bondarenko, N Eugeniy, Ostapchuk,O Oleksandr, Grygorenko, V Oleg, Shishkin, A Andrey, Tolmachev, Tetrahedron letters, 2010, 51, 4229-5232.
- Hai-Ming Guo, WU Yan-Yan, Hong-Ying Nill, Dong-Chao Wang, and QuGui-Rong, J Org Chem, 2010, 75,3863-3866.
- M.Suiko and K.Maekawa, Agric.Biol.Chem., 1977,41, 2047.
- M.Suiko, E.Taniguchi, K.Maekawa, and M.Eto, Agric.BioL Chem., 1979, 43,741.
- M.Suiko, E.Taniguchi, K.Maekawa, and M.Eto, BioLChem., 1979, 43, 747.
- S.Sh.Shukurov, M.A.Kukaniev, I.M.Nasyrov, L.S.Zakharov, and R.A.Kamkhanov, Zh.Obshch.ghim., 1993,63, 2320 [Russ.J.Gen.Chem., 1993, 63 (Engl. Transl.)].
- S.Sh.Shukurov, M.A.Kukaniev, 1.M.Nasyrov, L.S.Zakharov, and R.A.Karakhanov, lay.Akad.Nauk, Set.Khim., 1994, 908 [Russ.Chem.BAIL, t994, 43, 854 (Engl.Transl.) L.
- Pat.2712932, Germany, RZhKhim., 1980, 9.0.171P (Russ.Transl.).
- Pat.4742063 USA, RZhKhim., 1989, 1.0.401P (in Russian).
- Pat.4866064 USAI RZhKhim., 1991, 7.0384P (in Russian).
- M.Suiko and K.Maekawa, Agric.Biol.Chem., 1977,41, 2042.
- Y.Tominaga, J.Heterocycl.Chem., 1989, 26, 1167.
- H.Junjappa, H.Ila, and C.V.Asokan, Tetrahedron, 1990,46, 5423.

This work is licensed under a Creative Commons Attribution 4.0 International License.









