Biosuperabsorbent Hydrogel Based on Alginate-g-poly AA/kaolin Composite for Releasing Cefalexin Drug
Mohammad Sadeghi*, Fatemeh Shafiei And Esmat Mohammadinasab
Department of Chemistry, Science Faculty, Islamic Azad University, Arak Branch, Arak, Iran,
DOI : http://dx.doi.org/10.13005/ojc/300136
Article Received on :
Article Accepted on :
Article Published : 06 Feb 2014
To following synthesis of a novel alginte-based composite superabsorbent hydrogel was through crosslinking graft copolymerization of acrylic acid onto alginate in presence kaolin, we investigated the usefulness of a pH-sensitive protein-based hydrogel for in vitro controlled release of cefalexin. The loading drug yield was found to depend on the incubation time, the amount of encapsulated drug. In addition, the release of cefalexin from this kind of hydrogel was studied. The release rate of cefalexin from hydrogel at pH 7.4 was faster than that at pH 1.6 due to the shrinkage of the hydrogel at pH 1.6.
KEYWORDS:alginte; releasing drug; hydrogels; composite; cefalexin.
Download this article as:| Copy the following to cite this article: Sadeghi M, Shafiei F, Mohammadinasab E. Biosuperabsorbent Hydrogel Based on Alginate-G-PolyAA/kaolin Composite for Releasing Cefalexin Drug. Orient J Chem 2014;30(1) |
| Copy the following to cite this URL: Sadeghi M, Shafiei F, Mohammadinasab E. Biosuperabsorbent Hydrogel Based on Alginate-G-PolyAA/kaolin Composite for Releasing Cefalexin Drug. Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=2029 |
INTRODUCTION
Composite hydrogels have been of interest to biomaterial scientists for many years because of their hydrophilic character and potential to be biocompatible [1-2]. These networks are special soft and flexible polymeric materials that can absorb large quantities of water, saline or physiological solutions while the absorbed solutions are not removable even under pressure [4].
To date, many types of hydrogels as drug carriers have been widely investigated. Some interesting drug delivery systems based on hydrogels have thus been proposed [5-7]. Among hydrogels, however, considerable research attention has been focused on so-called “smart” hydrogels which can transfer their volume in response to environmental stimuli, and these results in environmental stimuli modifying drug release. Among these, pH-sensitive hydrogels have been extensively investigated for potential use in site-specific delivery of drugs to specific regions of the gastrointestinal tract and have been prepared for delivery of low molecular weight drugs. Therefore, these hydrogels have important applications in the field of medicine, pharmacy, and biotechnology.Controlled or sustained release drugs provide many advantages in comparison with conventional forms including reduced side effects, drug concentration kept at effective levels in plasma, improved utilization of drug and decrease the dosing times [9-11]. In the current study, we investigated utility of an anionic composite hydrogel based on alginate, for the controlled release of a beta-lactam antibiotic cephalosporin, cefalexin. Drug absorption and release capacities of
hydrogel systems and influence of pH of the medium on the release properties were also examined.
EXPERIMENTAL
Materials
Sodium alginate (chemical grade, MW 50000) ,N,N‘-methylene bisacrylamide (MBA, from Fluka), ammonium persulfate (APS, from Fluka), acrylic acid (AA, from Merck) with analytical grade were purchased and used without further purification. The drug, cefalexin, was obtained from Jaberebne Hayan Pharmaceutical Co. (Tehran, Iran). The chemical structure of cefalexin is shown in Figure 1. All other chemicals were also analytical grade. Double distilled water was used for the hydrogel preparation and swelling measurements.
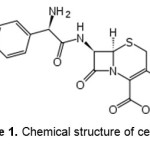 |
Figure 1. Chemical structure of cefalexin. |
Preparation of hydrogel
Synthesis of the hydrogel, H-alginte-g-PAcA/kaolin, was carried out using APS as an initiator and MBA as a crosslinker in an aqueous medium. A general procedure for crosslinking graft copolymerization of AcA onto alginte was conducted as follows. alginate (0.50-1.50 g) was added to a three-neck reactor equipped with a mechanical stirrer (Heidolph RZR 2021, three blade propeller type, 300 rpm), including 40 mL doubly distilled water. The reactor was immersed in a thermostated water bath preset at desired temperature (35-70 oC). After complete dissolution of alginte, various amounts of kaolin powder (0.25-0.75 g) were added to the alginte solution and allowed to stir (300 rpm) for 15 min. Then the initiator solution (0.01-0.40 g APS in 5 mL H2O) were added to the mixture. After stirring for 10 min, certain amounts of 70% neutralized AcA (2.0-8.0 g in 5 mL H2O) and MBA (0.05-0.20 g in 5 mL H2O) were simultaneously added to the reaction mixture. After 60 min, the produced hydrogel was poured to excess non-solvent ethanol (200 mL) and remained for 3 h to dewater. Then ethanol was decanted and the product scissored to small pieces (diameter ~5 mm). Again, 100 mL fresh ethanol was added and the composite hydrogel was remained for 24 h. Finally, the filtered composite is dried in oven at 60 oC for 10 h. After grinding, the powdered superabsorbent was stored away from moisture, heat and light.
Drug loading on hydrogels
The hydrogels were incubated with cefalexin using a contact adsorption technique. The swollen composite sample was dried in vacuum overnight until its weight remained unchanged. The vacuum dried powdered samples (1±0.0001 g), with average particle size in between 250–350 , were accurately weighted and placed in the aqueous solution of cefalexin (0.5 g dissolved in 50 mL distilled water) at 0oC for 48h to reach the equilibrium state. The swollen composite hydrogels loaded with cefalexin were placed in a vacuum oven and dried under vacuum at 37oC.
In vitro drug release
The samples (0.1±0.0001 g) were placed into 50 mL of the release medium (simulated gastric and intestinal fluids, SGF and SIF, respectively) at different pH values (pH 1.6 or 7.4) at 37oC with agitation. At fixed time intervals, 1 mL of the release medium was removed using a syringe attached with a 0.45 Millipore filter and after suitable dilution, the concentration of released cefalexin was measured spectrophotometrically at 265 nm.
RESULTS AND DISCUSSION
Effect of pH on equilibrium swelling
Ionic composit hydrogels exhibit swelling changes at a wide range of pHs. Therefore, in this series of experiments, equilibrium swelling for the synthesized hydrogels was measured in different pH solutions ranged from 1.0 to 13.0 (Figure 2). Since the swelling capacity of all “anionic” hydrogels is appreciably decreased by addition of counter ions (cations) to the swelling medium, no buffer solutions were used. Therefore, stock NaOH (pH 13.0) and HCl (1.0) solutions were diluted with distilled water to reach desired basic and acidic pHs, respectively. Maximum swelling (98 g/g) was obtained at pH 8. Under acidic pHs (≤4), most of the carboxylate anions are protonated, so the main anion-anion repulsive forces are eliminated and consequently swelling values are decreased. However, some sort of attractive interactions (H-O hydrogen bonding) lead to decreased absorbencies[12-14].
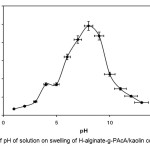 |
Figure 2. Effect of pH of solution on swelling of H-alginate-g-PAcA/kaolin composite hydrogel. Click here to View Figure |
At higher pHs (5-8), some of carboxylate groups are ionized and the electrostatic repulsion between COO– groups causes an enhancement of the swelling capacity. The reason of the swelling-loss for the highly basic solutions (pH>8) is “charge screening effect” of excess Na+ in the swelling media, which shields the carboxylate anions and prevents effective anion-anion repulsion. Similar swelling-pH dependencies have been reported in the case of other hydrogel systems[15].
Calibration curve
The calibration curve of the absorbance as a function of the cefalexin concentration at 265 nm, shown in Figure 3, has a linear relationship with correlation coefficients (r) of 0.996 and 0.998 at pHs 1.6 and 7.4, respectively.
 |
Figure 3. The standard spectrophotometric calibration curves of cefalexin at 266 nm and at pH 1.6 (a) and pH 7.4 (b). Click here to View Figure |
The cefalexin loading
The amount of cefalexin content entrapped in the hydrogels was determined by an indirect method. After the gel preparation, the washing solutions were collected, filtered with a 0.45 Millipore filter and tested at λmax 265 nm using the standard calibration curve of the drug recorded by UV/VIS spectrophotometer (UV-1201, Shimadzu, Kyoto, Japan).
The entrapped drug exhibited the same λmax as free drug. This clearly indicates that the entrapped drugs have not undergone any possible chemical reaction during the matrix formation. The difference in between initial drug and the drug content in the washing solutions is taken as an indication of the amount of entrapped drug:
For the investigation of drug adsorption behavior of hydrogels prepared in this study, hydrogels were firstly swollen in cefalexin solution in concentration range 0.50–3.0 mg/mL. As can be seen from the Figure 4, an increase in drug concentration in the swelling medium increased the amount of adsorbed drug, as observed in many adsorption studies [16-17]. This result can also be obtained from the following equation: 
where qe is in mg adsorbate per gram of dry adsorbent, Ci and C are the initial and equilibrium concentrations of adsorbate solution in mg.ml-1, Vt is the volume of solution treated in mL, and m is the mass of dry adsorbent, in g. As can be seen from Eq. 2, an increase in concentration of drug in the gel system increased with qe values.
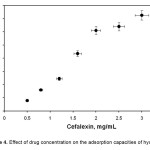 |
Figure 4. Effect of drug concentration on the adsorption capacities of hydrogel. |
The amounts of the loaded drug in superabsorbent hydrogels was also significantly affected by the incubation times (Figure 5). It is obvious that with increasing the loading time, the amount of drug loaded increased and then in the beginning decreased. The initial increment in the amounts of the loaded drug with increasing loading time can be ascribed to the increased drug diffusion into the swollen matrix[18]. The most effective time of loading efficiency was 30 h, where the high amount of drug was encapsulated.
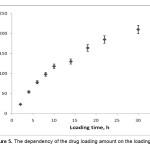 |
Figure 5. The dependency of the drug loading amount on the loading time. Click here to View Figure |
In vitro release behavior of hydrogels
To determine the potential application of gelatin-based superabsorbent containing a pharmaceutically active compound, the drug release behavior form this system under physiological conditions was investigated. The percentage of released drug from the polymeric carriers as a function of time is shown in Figure 6. The concentration of cefalexin released at selected time intervals was determined spectrophotometrically. The drug-loaded hydrogels with high drug loading (>83%) were prepared by the swelling-diffusion method. The amount of cefalexin released in a specified time from the alginate-based composite hydrogel decreased as the pH of the dissolution medium was lowered . At low pH values, electrostatic repulsion between the carboxylic acid groups of backbone is low, thus decreases gel swelling and minimizes release of cefalexin via diffusion. However, in alkaline media the presence of OH– increases the electrostatic repulsion between carboxylate groups, thus increases the gels, swelling degree and so the release of cefalexin increased [18,19].
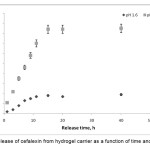 |
Figure 6. Release of cefalexin from hydrogel carrier as a function of time and pH at 37oC. |
CONCLUSION
In this research, after synthesis of composite hydrogel based on alginate, the loading and release of cefalexin from the pH-sensitive hydrogels was investigated. The results showed that the drug loading efficiency was decreased with increasing crosslinker content. The release value of cefalexin from hydrogels at pH 7.4 was higher than that at pH 1.6 due to the electrostatic repulsion between carboxylate groups. The hydrogels presented in this study may serve as a platform for a wide range of pharmaceutical uses to improve the bioavailability of beta-lactam antibiotic cephalosporin drugs.
References
- Raju, K. M.; Raju, M. P.; Mohan, M. P. J Appl Polym Sci ,2000, 85, 1795.
- Lim, D. W.; Yoon, K. J.; Ko, S. W. J Appl Polym Sci, 2000, 78, 2525.
- Buchholz F. L.; Graham, A. T.; Modern Superabsorbent Polymer Technology, Wiley, New York, 1997.
- Dai Y, Li P, Wang A. (2007) Intelligent drug delivery system of intelligent high polymer materials. Prog Chem 19: 362–369.
- Wu W, Wang D. (2010) A fast pH-responsive IPN hydrogel: Synthesis and controlled drug delivery. React Funct Polym 70: 684–691.
- Brandl F, Kastner F, Gschwind RM, Blunk T, Teßmar J, Göpferich A. (2010) Hydrogel-based drug delivery systems: Comparison of drug diffusivity and release kinetics. J Cont Rel 142: 221–228.
- Desai KGH, Park HJ. (2005) Preparation and characterization of drug-loaded chitosan-tripolyphosphate microspheres by spray drying. J Microencapsul 22(4): 377-395.
- Zohuriaan-Mehr MJ, Pourjavadi A. (2003) Superabsorbent hydrogels from starch-g-PAN: effect of some reaction variables on swelling behavior. J Polym Mater 20: 113-120.
- Jenkins DW, Hudson SM. (2001) Review of vinyl graft copolymerization featuring recent advances toward controlled radical-based reactions and illustrated with chitin/chitosan trunk polymers. Chem. Rev. 101: 3245-3273.
- Sen M, Yakar A. (2001) Controlled release of antifungal drug terbinafine hydrochloride from poly(N-vinyl 2-pyrrolidone/itaconic acid) hydrogels. Int J Pharm 228: 33-41.
- Peppas, L. B.; Harland, R.S. Absorbent Polymer Technology; Elsevier, Amsterdam, 1990.
- Pourjavadi A, Harzandi AM, Hosseinzadeh H. Eur. Polym. J.; 2004,40:1363.
- A. Pourjavadi, M. Sadeghi, H. Hosseinzadeh. Polym. Adv. Tech,2005.22,43-56 .
- Badiger, M. V.; McNeil, M. E.; Graham, N. B. Biomaterials, 1993,14, 1059-1063.
- Silverstein RM, Webster FX. Spectrometric Identification of Organic Compounds, 6th Edn., Wiley, New York, 1998.
- M. Sadeghi and H. Hosseinzadeh,Turk. J. Chem. 2010,34 ,739-752.
- Chen J, Zhao Y. Relationship,.J Appl Polym Sci; 2000,75:808.
- Hsu S.C, Don T.M, Chiu W.Y. Polym Degrad Stab; 2002,75:73.
- Lim DW, Whang HS, Yoon KJ. J. Appl. Polym. Sci.; 2001,79:1423.

This work is licensed under a Creative Commons Attribution 4.0 International License.









