An Efficient Method For The One-Pot, Four-Component Benzaldehyde Based Synthesis of 3-Methyl-1,4-Diphenyl-7,8-Dihydro-1H-Furo[3,4-E]Pyrazolo[3,4-B]Pyridin-5(4H)-Ones Catalyzed by Alum in Environment-Friendly Media.
Sadif A. Shirvan1*,Fereydoon Khazali1, Sara Haydari Dezfuli1 And Ali Borsalani2
1Department of Chemistry, Omidiyeh Branch, Islamic Azad University, Omidiyeh, Iran 2Department of Gas Engineering, Omidieh Branch, Islamic Azad University, Omidieh, Iran
DOI : http://dx.doi.org/10.13005/ojc/300135
Article Received on :
Article Accepted on :
Article Published : 30 Mar 2014
A mild and efficient method for the synthesis of 3-methyl-1,4- diphenyl-7,8-dihydro-1H-furo[3,4-e]pyrazolo[3,4-b]pyridin-5(4H)-one derivatives via one-pot, four-component reaction of aromatic aldehydes, tetronic acid, 3-aminobut-2-enenitrile, and phenylhydrazine is described using Alum as catalyst. The features of this procedure are mild reaction conditions, excellent yields, short reaction time, and operational simplicity.
KEYWORDS:Alum; Multicomponent Reactions (MCRs); Ionic liquids; benzaldehyde
Download this article as:| Copy the following to cite this article: Shirvan A. S, Khazali F, Dezfuli H. S, Borsalani A. An Efficient Method For The One-Pot, Four-Component Benzaldehyde Based Synthesis of 3-Methyl-1,4-Diphenyl-7,8-Dihydro-1H-Furo[3,4-E]Pyrazolo[3,4-B]Pyridin-5(4H)-Ones Catalyzed by Alum in Environment-Friendly Media. Orient J Chem 2014;30(1). |
| Copy the following to cite this URL: Shirvan A. S, Khazali F, Dezfuli H. S, Borsalani A. An Efficient Method For The One-Pot, Four-Component Benzaldehyde Based Synthesis of 3-Methyl-1,4-Diphenyl-7,8-Dihydro-1H-Furo[3,4-E]Pyrazolo[3,4-B]Pyridin-5(4H)-Ones Catalyzed by Alum in Environment-Friendly Media.. Orient J Chem 2014;30(1). Available from: http://www.orientjchem.org/?p=2587 |
INTRODUCTION
Application of multicomponent reactions (MCRs) to the construction of natural product-based libraries would be most beneficial to this area of research. Such processes in which three or more reactants are combined together in a single reaction flask to produce a product. Up to seven starting components have been used, and MCRs have often been shown to produce higher product yields than classical chemistry. Multi-component reactions (MCRs) have emerged as an efficient and powerful tool in modern synthetic organic chemistry due to their valued features such as atom economy, simpler procedures and equipment, time and energy savings, straightforward reaction design, and the opportunity to construct target compounds by the introduction of several diversity elements in a single chemical event. MCRs, leading to interesting heterocyclic scaffolds, are particularly useful for the construction of diverse chemical libraries of ‘drug-like’ molecules. 1-8 Tetronic acid (tetrahydrofuran-2,4-dione) is a promising convenient building block and compoundsin which tetronic acid fragment is fused to a heterocyclic systems attract specific attention. A large number of tetronic acid derivatives are widespread in nature found in sponges, lichens, and molds, as well as in higher plants. Many exhibit a wide array of biological properties such as antibiotic, anticoagulant, antiepileptic, antifungal, insecticidal, analgesic and anti-inflammatory activities. Recently, these compounds have also been reported as HIV-1 protease inhibitors. 9-19 Polyfunctionalized heterocyclic compounds play important roles in the drug discovery process, and analysis of drugs in late development or on the market shows that 68% of them are heterocycles. Therefore, it is not surprising that research in the field of synthesis of polyfunctionalized heterocyclic compounds has received special attention.4 Ionic liquids (ILs) have received vast research interests in recent years. They exhibited unique properties such as high ionic conductivity, wide electrochemical window, non-volatility, high thermal stability, nonflammability and miscibility with organic compounds, especially with the heterocyclic compounds as hydrophilicity, hydrophobicity, Lewis acidity, viscosity and density can be altered by the fine-tuning of parameters such as the choice of organic cation, inorganic anion and the length of alkyl chain attached to the organic cation (Fig. 1). 20-23Because of these useful properties, ILs have been applied in several areas, including catalysis, electrochemistry, separation science for extraction of heavy metal ions, as solvents for green chemistry and materials for optoelectronic applications. 24-28 Room temperature ionic liquids are increasingly finding a range of laboratory, developmental and technical applications, for example, as media for organic and inorganic chemical synthesis, materials productions, and electrochemical devices21. As a part of our research program, which aims to develop new and environmentally friendly methodologies for the preparation of heterocyclic compounds, 29 recently, synthesis of 3-methyl-1,4-diphenyl-7,8-dihydro-1H-furo[3,4-e]pyrazolo[3,4-b]pyridin-5(4H)-ones was reported via three-component reaction, 30 herein, we report a simple and efficient method for the synthesis of these compounds with fused furo, pyrido and pyrazolo rings, through the one-pot four-component reaction in green media, using Alum as a catalyst at short reaction times with excellent yields.
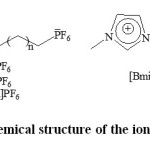 |
Figure: Chemical structure of the ionic liquids Click here to View Figure |
EXPRIMENTAL
General remark
All chemicals were purchased from commercial providers and were used as received.
Physical measurements
The progress of the reaction was monitored by1H NMR spectra were recorded on a BRUKER DRX-300 AVANCE spectrometer at 300.13 MHz, Elemental analyses were performed using a Heracus CHN-O-Rapid analyzer, FT-IR on Nicolet 400D and TLC.
Synthesis, Spectral and Analytical Data of Some Representative Compounds.
A mixture of tetronic acid (1 mmol), 3-aminobut-2-enenitrile (1 mmol) , phenylhydrazine (1 mmol) and Catalyst (0.05 g) in ionic Liquid (0.2 g) was stirred for 5 min at 70 °C. Then benzaldehyde (1.2 mmol) was added to the mixture and stirred for 15 min at this temperature. After cooling, the water (10 ml) was added to the reaction mixture and filtered. The precipitate washed with water (10 mL) and EtOH (10 mL) to afford the pure product.
6,7-dihydro-4,9-diphenylfuro[3,4-b]quinoline-1,8(3H,4H,5H,9H)-dione (1a): IR(KBr, v,cm-1): 1737, 1674;1H NMR (300 MHz, DMSO-d6): δH (ppm) 1.69-1.89 (m, 2H, CH2), 2.14-2.23 (m, 2H, CH2), 2.17-2.28 (m, 2H, CH2), 4.56-5.02 (m, 2H, CH2),4.73 (s, 1H, CH), 6.88-7.80 (m, 10H, H-Ar). Anal. Calcd for C23H19NO3: C, 77.29; H, 5.36; N, 3.92. Found C, 77.37; H, 5.24; N, 3.49.
9-(4-bromophenyl)-4-phenyl-5,6,7,9-tetrahydrofuro[3,4-b]quinoline-1,8(3H,4H)-dione (4e):IR(KBr, v,cm-1): 1751, 1667;1H NMR (300 MHz, DMSO-d6): δH (ppm) 1.73-1.92 (m, 2H, CH2), 2.13-2.24 (m, 2H, CH2), 2.28-2.34 (m, 2H, CH2), 4.53-4.59 (m, 2H, CH2),4.89 (s, 1H, CH), 6.88-7.80 (m, 9H, H-Ar). Anal. Calcd for C23H18BrNO3: C, 63.32; H, 4.16; N, 3.21. Found C, 63.37; H, 4.20; N, 3.16.
9-(4-methoxyphenyl)-4-p-tolyl-5,6,7,9-tetrahydrofuro[3,4-b]quinoline-1,8(3H,4H)-dione (4f):IR(KBr, v,cm-1): 1756, 1678;1H NMR (300 MHz, DMSO-d6): δH (ppm) 1.71-1.86 (m, 2H, CH2), 2.10-2.23 (m, 2H, CH2), 2.26-2.33 (m, 2H, CH2), 2.41 (s, 3H, CH3), 3.75 (s, 3H, OCH3), 4.50-4.58 (m, 2H, CH2), 4.61 (s, 1H, CH), 6.64-7.76 (m, 8H, H-Ar). Anal. Calcd for C25H23NO4: C, 74.79; H, 5.77; N, 3.49. Found C, 74.74; H, 5.81; N, 3.45.
9-(4-methoxyphenyl)-6,6-dimethyl-4-phenyl-5,6,7,9-tetrahydrofuro[3,4-b]quinoline-1,8(3H,4H)-dione (4h):IR(KBr, v,cm-1): 1745, 1689;1HNMR (300 MHz, DMSO-d6): δH (ppm) 0.84 (s, 3H, CH3), 0.95 (s, 3H, CH3), 2.02-2.08 (m, 2H, CH2), 2.17-2.23 (m, 2H, CH2), 3.70 (s, 3H, OCH3), 4.41-4.54 (m, 2H, CH2),4.68 (s, 1H, CH), 6.85-7.81 (m, 9H, H-Ar). Anal. Calcd for C26H25NO4: C, 75.16; H, 6.06; N, 3.37. Found C, 75.12; H, 6.11; N, 3.33.
9-(4-bromophenyl)-6,6-dimethyl-4-p-tolyl-5,6,7,9-tetrahydrofuro[3,4-b]quinoline 1,8(3H,4H)-dione (4k): IR(KBr, v,cm-1): 1750, 1683;1H NMR (300 MHz, DMSO-d6): δH (ppm) 0.82 (s, 3H, CH3), 0.91 (s, 3H, CH3), 2.00-2.05 (m, 2H, CH2), 2.16-2.20 (m, 2H, CH2), 2.40 (s, 3H, CH3), 4.45-4.52 (m, 2H, CH2), 4.65 (s, 1H, CH), 6.65-7.68 (m, 8H, H-Ar). Anal. Calcd for C26H24BrNO3: C, 65.28; H, 5.06; N, 2.93. Found C, 65.31; H, 5.10; N, 2.90.
RESULTS AND DISCUSSION
In our first attempt, four-component reaction of benzaldehyde, 1a tetronic acid, 2 3-aminobut-2-enenitrile, 3 and phenylhydrazine, 4 was done in various conditions. Additionally, different types and amounts of catalysts were considered in order to optimize the reaction conditions. We were pleased to note the formation of 5a in excellent yield (98%). At first, we examined this simple model reaction in the presence of a diverse types of lewis and bronsted acids such as Alum, SSA, SnCl4, ZnCl2, SnO2, p-TSA, CAN, and K-10 as a catalyst. We also tested the reaction in different ionic liquids media such as [Bmim]Br, [Bmim]Cl, [Bmim]CF3COO, [Bmim]BF4, and [Bmim]PF6. It was found that only trace amount of the target compound 5a was obtained in the absence of catalyst. We have found that use of Alum has a unique capability and best promoter to enhance the reaction rate in [Bmim]PF6 medium (Scheme 1).
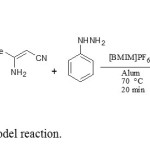 |
Scheme1: Standard model reaction. Click here to View Scheme |
We also evaluated the amount of catalyst needed for this conversion. It was found that using 10 mol % Alum in ionic liquid is adequate to push the reaction forward. There isn’t any increasing in the reaction yield by more amount of the catalyst. However, use of 10 mol % of SSA and p-TSA led to lower yields (60–70%) after 60 min reaction time. The best results were obtained by Alum as catalyst and [Bmim]PF6 at 70 ºC(Schem 2). The consequences are summarized in Table 1.
![Schem 2: synthesis of 3-methyl-1,4-diphenyl-7,8-dihydro-1H-furo[3,4-e]pyrazolo[3,4-b]pyridin-5(4H)-ones](http://www.orientjchem.org/wp-content/uploads/2014/03/Vol30_No1_efficie_SADI_sch2-150x150.jpg) |
Schem 2: synthesis of 3-methyl-1,4-diphenyl-7,8-dihydro-1H-furo[3,4-e]pyrazolo[3,4-b]pyridin-5(4H)-ones |
Table 1: Model Reaction, Conditions, and Yields
|
Conditions |
Catalyst |
Time (min) |
T.(°C) |
Yield(%) |
|
[Bmim]PF6 |
CAN |
20 |
70 |
<50 |
|
[Bmim]PF6 |
p-TSA |
20 |
70 |
66 |
|
[Bmim]PF6 |
SnCl4 |
20 |
70 |
<50 |
|
[Bmim]PF6 |
SSA |
20 |
70 |
63 |
|
[Bmim]PF6 |
Alum |
20 |
70 |
98 |
|
[Bmim]PF6 |
ZnCl2 |
20 |
70 |
<50 |
|
[Bmim]PF6 |
SnO2 |
20 |
70 |
<50 |
|
[Bmim]BF4 |
Alum |
20 |
70 |
88 |
|
[Bmim]CF3CO2 |
Alum |
20 |
70 |
91 |
|
[Bmim]Br |
Alum |
20 |
70 |
70 |
|
[Bmim]Cl |
Alum |
20 |
70 |
61 |
After optimizing the conditions, to delineate this approach, particularly in regard to library construction, as shown in Table 2 it was found that this procedure works with a wide variety of benzaldehydes. Corresponding 3-methyl-1,4-diphenyl-7,8-dihydro-1H-furo[3,4-e]pyrazolo[3,4-b]pyridin-5(4H)-ones 5 were synthesized by the one-pot, four-component condensation of benzaldehydes, 1(a-l) tetronic acid, 2 3-aminobut-2-enenitrile, 3 and phenylhydrazine, 4 with excellent yields in the ionic liquid media in the presence of Alum as a catalyst for 20 min at 70 °C . Work-up gave products 5(a-l) 93% to 98% yields. The results are summarized in Table 2. All the products 5(a-l) are Known compounds and were characterized by comparison of their melting point data with authentic samples synthesised by reported procedures30. a.Isolated Yields b.Melting point data in authentic samples synthesised by reported procedures 30. Subsequently, another active carbonyl compounds, indoline-2,3-dione and acenaphthylene-1,2-dione, were examined as the replacement of aromatic aldehydes in the reaction with tetronic acid, 3-aminobut-2-enenitrile, and phenylhydrazine, (Scheme 3).
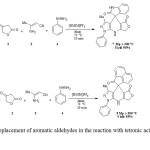 |
Scheme 3: Replacement of aromatic aldehydes in the reaction with tetronic acid, 3-aminobut-2- enenitrile Click here to View Scheme |
Table 2: 4,9-diphenyl-5,6,7,9-tetrahydrofuro[3,4-b]quinoline-1,8(3H,4H)-dione derivatives5
|
Entry |
5 |
R |
Yielda (%) |
Mp ºC |
Lit.b Mp ºC |
|
1 |
a |
4-CH3C6H4 |
98 |
224-225 |
222-224 |
|
2 |
b |
4-BrC6H4 |
93 |
240-242 |
238-240 |
|
3 |
c |
4-OHC6H4 |
98 |
259-262 |
358-260 |
|
4 |
d |
3,4-OCH2OC6H3 |
95 |
270-272 |
268-270 |
|
5 |
e |
4-CH3OC6H4 |
96 |
233-235 |
230-232 |
|
6 |
f |
4-ClC6H4 |
97 |
222-223 |
223-224 |
|
7 |
g |
4-FC6H4 |
96 |
250 |
249-250 |
|
8 |
h |
3-ClC6H4 |
98 |
169-172 |
168-170 |
|
9 |
i |
4-NO2C6H4 |
98 |
230-231 |
228-230 |
|
10 |
J |
3,4-Cl2C6H3 |
97 |
243-244 |
244-246 |
|
11 |
K |
2-ClC6H4 |
96 |
259-260 |
258-260 |
|
12 |
L |
2,4-Cl2C6H3 |
97 |
257 |
254-256 |
Scheme 3: Replacement of aromatic aldehydes in the reaction with tetronic acid, 3-aminobut-2- enenitrile
In these reactions 3-methyl-1-phenyl-7,8-dihydrospiro[furo[3,4-e]pyrazolo [3,4-b]pyridine-4,3′-indoline]-2′,5(1H)-dione and 3′-methyl-1′-phenyl-7′,8′-dihydro-2H-spiro[acenaphthylene-1,4′-furo[3,4-e]pyrazolo[3,4-b]pyridine]-2,5′(1′H)-dione were synthesized with excellent yields in the same conditions.
Conclusion
In conclusion, a facile and green one-pot four component procedure for the syntheses of 3-methyl-1,4-diphenyl-7,8-dihydro-1H-furo[3,4-e]pyrazolo[3,4-b] pyridin-5(4H)-ones in short reaction time is reported. Using readily available starting materials and Alum as a inexpensive and nontoxic catalyst in environmentally friendly reaction media, operational simplicity, excellent product yields and easy work-up procedures are prominent among the advantages of this new method.
Acknowledgements
We gratefully acknowledge financial support from the Research Council of Islamic Azad University, Omidieh Branch.
References
- Multicomponent Reactions, J. Zhu, H. Bienayme, Eds. Wiley-VCH. Weinheim 2005.
- A. Basso, L. Banfi, R. Riva, G. Guanti, J. Org. Chem., 70,575( 2005).
- D. J. Ramon, M. Yus,Angew. Chem., 44, 160(2005).
- A .Domling, Chem. Rev., 106, 17(2006).
- I .Ugi, S. Heck, Comb. Chem. High Throughput Screening., 4, 1(2001).
- M. Shigemitsu, Bull. Chem.Soc.Jpn., 32,692(1959).
- R. Gharamanzadeh, A. Bazgir, J.Hetrocycl.Chem., 47,421(2010).
- R. Gharamanzadeh, S. Ahadi, A. Bazgir, J.Hetrocycl.Chem., 51,449(2010).
- N. K. Satti, A. K.Suri, O .P. Sun, Indian.J.Chem B., 32.978(1993).
- F. Effenberg, J. Syed, Tetrahedron: Asymmetry., 9, 817(1998).
- L. J.Haynes, Plimmer, J. R. Quart. Rev., 14, 292(1960).
- S.V. Ley, M. L. Trudell, D. Wad worth, Tetrahedron., 47, 8285(1991).
- B. E. Vanwagenen, J. H.Cardellina, Tetrahedron., 42, 1117(1986).
- R. A.Vishwakarma, R. S .K apil, S. P.Popli, Indian J. Chem., 26, 486(1987).
- K. Luk, S. A. Readshaw, J. Chem. Soc., 11,1641(1991).
- A. Ibi, E.Yaniguchi, K. Maekawa, Agric. Biol. Chem., 43, 1641(1979).
- R. Brodersen, A. Kjaer, Acta Pharmacol. Toxicol., 2, 109 (1946).
- F. R.Foden, J.McCormick, D. M.O’Man.,J. Med. Chem., 18, 199(1975).
- B. E. Roggo, F. Petersen, R. Delmendo, H. B. Jenny, H. H. Peter, J. Roesel, J. Antibiot., 47, 136(1994).
- R. Hagiwara, Y. Ito, J. Fluorine Chem., 105, 221(2000).
- T. Welton, Chem. Rev., 99, 2071(1999).
- M. J. Earle, K. R. Seddon, Pure Apple. Chem., 72, 1391(2000).
- H. Olivier, J. Mol. Catal. A. Chem., 146, 285(1999).
- J. H. Davis, K. J. Forrester, T. Merrigan, Tetrahedron Lett., 39, 8955(1998).
- J. G Huddleston, H. D. Willauer, R. P. Swatloski, A. E. Visser, R. D, Rogers, Chem. Commun., 1765(1998).
- A. Rosowsky, C. E. Mota, S. F. Queener, J. hetrocyclic.Chem., 32,335(1995).
- A. E. Visser, R. P Swatloski, W. M. Reichert, R Mayton, S. Sheff,A. Davis, Chem. Commun., , 135(2001).
- D. Haristoy, D. Tsiourvas, Chem. Mater., 15, 2079(2003).
- S. A. Shirvan, R. Ghahremanzadeh, M. Mirhosseini Moghaddam, A. Bazgir, A. H. Zarnani, M. M. Akhondi, J. Heterocyclic Chem., 2011, (In Press).
- C. L. Shi, D. Q. Shi, S. H. Kim, Z. B. Huang, Tetrahedron., 64, 2425(2008).

This work is licensed under a Creative Commons Attribution 4.0 International License.









