Synthesis, Characterization and Biocidal Activity of some Schiff base and its Metal Complexes of Co(II), Cu(II) and Ni(II)
B. K. Rai* and Arun Kumar1
*Department of Chemistry, L. N. T. College, Muzaffarpur, B.R.A. Bihar University, Muzaffarpur 1Department of Chemistry, KCTC College, Raxaul, B. R. A Bihar University Muzaffarpur
DOI : http://dx.doi.org/10.13005/ojc/290349
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
A series of metal complexes derivatives of 1-propyl-2-6-diphenyl piperidone semicarbazone(PDPS) with metal ions Cu(II), Co(II) and Ni(II) have been synthesized. The ligand and metal complexes obtained are characterized quantitatively and qualitatively by using, molar mass, elemental analyses, Infrared spectra, electronic spectra, magnetic susceptibility and conductivity measurements. On the basis of above physiochemical analysis, it has been observed that the ligand PDPS coordinate to the metal ion in a bidentate manner through azomethine nitrogen and oxygen atom of semicarbozone moiety. The remaining coordination centers are satisfied by anions such as X = Cl-, Br- and I-. Electronic spectral and magnetic susceptibility measurement proposed the general composition of the complex is [M(PDPS)2X2] where M = Cu(II), X = Cl- and Br-; M = Co(II) and Ni(II), X = Cl-, Br- and I-. The complexes of Co(II) and Ni(II) were proposed octahedral geometries whereas distorted octahedral geometry reported for Cu(II) complexes. The preliminary in vitro antibacterial Screening activity revealed that complexes showed better inhibition against tested bacterial strains and higher compared to parent ligand.
KEYWORDS:PDPS;Semicarbazone;Cu(II);Co(II) and Ni(II)/complexes;antibacterial activity
Download this article as:| Copy the following to cite this article: Rai B. K, Kumar A. Synthesis, Characterization and Biocidal Activity of some Schiff base and its Metal Complexes of Co(II), Cu(II) and Ni(II). Orient J Chem 2013;29(3). doi : http://dx.doi.org/10.13005/ojc/290349 |
| Copy the following to cite this URL: Rai B. K, Kumar A. Synthesis, Characterization and Biocidal Activity of some Schiff base and its Metal Complexes of Co(II), Cu(II) and Ni(II). Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=360 |
INTRODUCTION
Wide varieties of geometries and reactivity of semicarbozene metal complexes have been reported1-7 to possess several biocidal activities. The chemical and biocidal activity of semicarbozone have been subject of investigation in recent years. The biological activity is related to their interaction with several metal ions. Schiff bases ligand and their metal complexes play an important application in the area of analytical chemistry, polymer sciences, Food and dyes industry, agriculture, biological sciences as antimicrobial agents, medical sciences as anticancer and metal corrosion inhibition agents. In view of the growing interest in the biocidal importance of Schiff and their metal complexes and in continuation8-12 of our earlier recent work in this field we now report, the synthesis, characterization and antibacterial activity of Cu(II), Co(II) and Ni(II) complexes with bidentate Schiff base, 1- propyl 2- 6- diphenyl piperidone semicarbazone (PDPS).
EXPERIMENTAL
All the chemicals and reagents used were analytical grade. The solvents were used without any purification. The metal contents were determined using standard procedure13. Physical and analytical data of the Schiff base and their complexes is given in Table-1 and agreed with proposed formulae. The complexes are coloured and insoluble in water and common solvents but sparingly soluble in DMF.
Preparation of the ligand
The ligand PDPS was synthesized by the condensation of 1-propyl 2-6-diphenyl piperidone with semicarbazide hydrochloride in 1:1 molar ratio using absolute alcohol as the reaction medium. The mixture was refluxed on a water bath for 2 and a half hour and then allowed to stand overnight at room temperature. The product were crystallized from the same solvent and dried in vacuum. M.P.-163+IoC. Yield=65%.
Preparation of the complexes
The complexes of Cu(II), Co(II) and Ni(II) have been prepared by reacting ethanolic solution of metal halides with ethanolic solutions of the ligand in the molar ratio 1:2. The solid coloured complexes which is separated on cooling were filtered, washed with ethanol, dried in oven. Yield in all cases 60%-65%.
RESULTS AND DISCUSSION
I.R. spectra
IR spectra of the ligand and complexes were recorded on Perkin Elmer Spectrophotometer Model 577 using KBr disc.
The IR spectra of the ligand exhibit strong and broad band at 1660 cm-1 assignable to nC=N. The band is shifted to lower wave numbers after complex formation proposes involvement of azomithine N in the banding with metal ions. The linkage with N atom is further supported by the appearance of a band in far IR region at 425-395cm-1 in the complexes assignable15 to nM–N. The IR spectra of the ligand exhibits strong and broad band at 1760 cm-1 assignable to nC=O. This band undergoes to shift after complex formation propose coordination of metal ion through carbohyl oxygen. It is further supported by the appearance of a new far IR band at 525-505cm-1 in the complexes which is assignable17 to nM–O. The linkage with halogen is indicated by the appearance of another band in the far IR region 320-280cm-1 assigned18 to nM–X (X = Cl–, Br– or I–). The coordination with halogen is supported by the low molar conductivity of the complex in the range 11.2-13.7 ohm-1cm2mol-1. The electronic spectral and magnetic measurements data proposes octahedral geometry for the complexes which is justified by other physiochemical as well as Infrared spectral data.
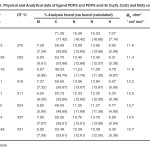 |
Table-1 Physical and Analytical data of ligand PDPS and its Cu(II), Co(II) and Ni(II) complexes Click here to View table |
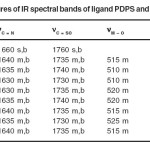 |
Table – 2: Salient features of IR spectral bands of ligand PDPS and its metal complexes Click here to View table |
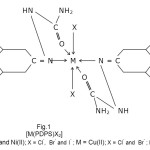 |
Fig.1 [M(PDPS)X2]M = Co(II) and Ni(II); X = Cl–, Br– and I– ; M = Cu(II); X = Cl– and Br– ; R = Phenyl |
Molar Conductance
Conductivity of the complexes were measured in the solvent DMF on Systronic conductivity meter modle 303 at the concentration 10-3 M and all the complexes were found to be non electrolytic19 in nature. The molar conductance measurements of the complexes were found in the range 11.4-13.7 ohm-1 cm2 mol-1. The conductivity value also supported the structure assigned on the basis of physiochemical and spectroscopic measurements.
Antibacterial Study
Schiff bases and their metal complexes have been reported antibacterial activity against the Escherichia coli and Staphylococcus aureus by using paper disc diffusion method.20 The result reveals that the metal chelates are more potent than the parent ligand. The increased potency of metal complexes may be attributed to their increased lipophillic nature arising due to chelation21,22 mode of action of antimicrobials due to involvement of various targets in microorganism.
Conclusion
Thus on the basis of elemental analyses, IR, electronic spectra, magnetic susceptibility, molar conductivity measurement studies it may be proposed that the ligand PDPS behaves as a neutral bidentate manner and coordination is proposed through azomethine nitrogen atom and carbonyl oxygen of semicarbazone moiety. The remaining coordination centres are satisfied by negative ions such as Cl-,,Br– or I-. On the basis of available evidence octahedral geometry has been proposed for Cu(II), Co(II) and Ni(II) complexes as shown in Fig-1.
REFERENCES
- Kaur M. and Singh A.K., Asian J. Chem.m 10 233(1998).
- Tai X.S., Yim X.H. and Tan M.Y., Polish J. Chem., 77, 411(2003).
- Adsule S., barve V., Chem P., Ahmed F., Dou Q.P., Padhye S. and Sarkar F.H., J. Med. Chem; 69, 7242(2006).
- Tian L.J., Yang H.G., Zheng X.L., Ni Z.H., Yan D.M., Tu L.L. and Jiang J.Z., Appl. Organometal Chem; 23, 24(2009).
- Hou J., Yang Y. and Zhu H.L., J. Chem., Crystallog., 40, 661(2010).
- Sun P.P., Jian F.F. and Wang X., J. Chem; Crystallorg.; 40, 4(2010).
- Zhang R.L., Zhao J.S., Yang P. and Shi Q.Z., J. Chem. Crystallog; 40, 357(2010).
- Rai B.K. and Anand Rahul, Asian J. Chem, 25, 480(2013).
- Rai B.K., Thakur Amrita and Divya, Asian J. Chem.; 25,, 583(2013).
- Rai B.K., Vidyarthi S.N., Kumari Punam, Kumari Suman, Kumari Lakshmi and Singh Rajkishore, Asian J. Chem., 25, 941(2013).
- Rai B.K. and Kumar Arun, Asian J. Chem., 25, 1169(2013).
- Rai B.K., J. Indian chem.; Soc., 90, 105(2013).
- Vogel A.I., A textbook of quantitative Chemical Analysis, Revised by Menaham J., Denny R.C., Barnes J.D. and Thomas M, Pearson Education, 7th Edn, London(2008).
- Singh N.K., Srivastave A.K. and Agarwal R.C., Indian J. Chem., 22A, 704(1984).
- Mahapatro B.B. , Sarat S.K., J. Indian Chem. Soc; 80, 696(2003).
- Singh P.P. and Shukla U.P., Inorg Chem; Acta, 7, 493(1973).
- Dash D.C. Behera R.K., Sen M. and Mehar F.M., J. Indian Chem. Soc; 80, 696(2003).
- Silverstein R.M. and Webster F.X., Spectrometric Identification of Organic Compounds, 6th edn, John Wiley and Sons, 109(2008). Kemp William, Organic Spectroscopy, Polgrave, New York, 3rd Edn.(2008).
- Kettle S.F.A., Coordination Compounds Thomas Nelson and Sons 168(1975).
- Bour A.W., Kirby W.M., Sherris J.E. and Turk M., Am. J. Clin. Pathol., 45, 493(1966).
- Fahmi M. and Singh R.V., Transition Met. Chem.; 15, 399(1972).
- Thornberry H.H., Phytophatology, 40, 411(1950).

This work is licensed under a Creative Commons Attribution 4.0 International License.









