Synthesis and Thermal Properties of Nickel B-diketonate Polymers
Mohammed A. Al-Anber
Department of Environmental Health, Faculty of Public Health and Health Informatics, Hail University, Hail, Saudi Arabia
DOI : http://dx.doi.org/10.13005/ojc/290301
Article Received on :
Article Accepted on :
Article Published : 28 Sep 2013
A mononuclear complex of [Ni(tta)2(H2O)2] (3) (tta = deprotonated of 1-thenoyl-4,4,4-trifluoroacetone (1)) has been prepared by the reaction of 1-thenoyl-4,4,4-trifluoroacetone (H-tta: 1) with Ni(OAc)2.4H2O (OAc = O2CMe) in a 2:1 molar ratio. Complex 3 can be extended to form a coordination polymers of general formula [Ni(tta)2(X)]n (X = 4,4'-bipy (4), pz (5), 1,4-dip (6)) by the reaction of nickel atom in 3 with s-donor ligand such as 4,4-bipyridine (4,4'-bipy), pyrazin (pz), and 1,4-diisocyanobenzene (1,4-dip). The reaction completion was controlled via FTIR and elemental analysis. ThermoGravimetric (TG) studies of 3 - 6 show a great thermal stability up to ca. 400 ºC for polymers, wherein the nickel oxide is formed at higher temperature as shown by calculations.
KEYWORDS:Nickel;4,4’-Bipyridine;Polymers;1-thenoyl-4,4,4-trifluoro-acetone,Pyrazine,ThermoGravimetric
Download this article as:| Copy the following to cite this article: Al-Anber M. A. Synthesis and Thermal Properties of Nickel B-diketonate Polymers. Orient J Chem 2013;29(3) |
| Copy the following to cite this URL: Al-Anber M. A. Synthesis and Thermal Properties of Nickel B-diketonate Polymers. Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=31 |
Introduction
Metal-organic polymers including b-diketonate fragments have attracted increasing interests as advanced materials due to their unique properties and multifunctionality [1]. The design and synthesis of such polymer chains can be constructed by binding of metal atom with σ-donor rod-like bridging ligands such as 4,4′-bipyridine and pyrazine [2]. The type of substituent groups on the b-diketonate fragments could spontaneously stack the polymeric chains into a large 1D-, 2D-, and 3D-supramolecular structure via non-covalent interactions [3-4]. It has been widely observed that such polymeric materials can be influenced by the choice of the metal ion and the bridging ligand species [5] as well as the type of non-covalent interactions between these chains.
A number of supermolecules including b-diketonates, such as 1-(2-furyl)-4,4,4-trifluoro-1,3-butanedione (Htfb), 1,1,1-trifluoro-2,4-pentanedione (H-tp), and 1-phenyl-4,4,4-trifluoro-1,3-butanedione (Htpb), with different metal centers, such as Cu(II), Mn(II), Ni(II), Zn(II) metal ions have been reported [6-12]. Recently, a similar supermolecules structures such as [UO2(tfb)2]n.OHCH3 (tfb = 1-(2-furyl)-4,4,4-trifluoro-1,3-butanedione) [13], [Co(tta)2(OHCH3)2]n [14], [Cu(tta)2]n (tta = 1-thenoyl-4,4,4-trifluoroacetone) [15] have been reported. The moving from the metal-organic supermolecule structures to the supramolecular 1D-coordination polymeric chains or 2D-network also have been reported [16]. Mostly, the 1D-polymeric chains were formed by reacting of simple cobalt salts with 4,4¢-bipyridine (4,4′-bipy) or pyrazine (pz) [17-20]. In this respect, three isomorphus 1D-coordination polymers of [Co(OAc)2(4,4′-bipy)]n (OAc = CH3COO), [Co(H2O)3(4,4′-bipy)SO4].2H2O and [Co(H2O)3(4,4′-bipy)Cl2].2H2O were synthesized and structurally characterized [17]. The solid-state structures for these polymers show one dimensional Co-bipy-Co chains. The polymeric chains of [Co(H2O)3(4,4′-bipy)SO4].2H2O are self-assembly stacked through hydrogen bonds producing a 2D-supramolecular network. Similarly, the polymeric structure of [Co(OAc)2(4,4′-bipy)]n contains linear double Co-bipy-Co chains bridged by CH3COO– groups. From another hand, the synthesis, structure, and reactivity of [Co(acac)(4,4′-bipy)]n [18] and [Co(acac)(pz)]n (acac = acetylacetone, pz = pyrazine) [16] polymeric chains were described. The Co atoms in these chains are coordinated in an elongated octahedral geometry with non coplanar of pyridine rings. Between these chains a weak van der Waals interactions were present. Therefore, they may be regarded as essentially 1D-chain structure not as 2D-network. In addition, another isomorphous structure to [Co(acac)(4,4′-bipy)]n [18] were reported using different metal atom such as [Cu(acac)(4,4′-bipy)]n [21]. Recently, the reaction of [Co(tta)2(H2O)2] [14] with one equivalents of 4,4′-bipyridine (4,4′-bipy) produces 1D-coordination polymer [Co(tta)(4,4′-bipy)]n [14], whereby the supramolecularity, structurally and thermally of [Co(tta)(4,4′-bipy)]n are characterized. These 1D-polymeric chains are stacked through the presence of S…S intermolecular interactions between thiophene rings of individual chains.
In this context, we have synthesized a mononuclear nickel complex of [Ni(tta)2(H2O)2] (3) and three kind of coordination polymers of [Ni(tta)2(X)]n (X = 4,4′-bipy (4), pz (5), 1,4-dip (6); tta = deprotonated of 1-thenoyl-4,4,4-trifluoroacetone) by the reaction of nickel atom in 3 with s-donor ligand such as 4,4-bipyridine (4,4′-bipy), pyrazin (pz), and 1,4-diisocyanobenzene (1,4-dip).
Experimental
General remarks
All chemicals were purchased from commercial providers (Fluka Company) and were used as received.
Physical measurements
Infrared spectra were recorded using a Perkin-Elmer FTIR 1000 spectrometer. Melting points were determined using analytically pure samples with a Gallenkamp MFB 595 010M melting point apparatus. Microanalyses were performed using a Thermo FLASHEA 1112 Series instrument. Thermogravimetric studies were carried out with the Perkin Elmer System Pyris TGA 6 with a constant heating rate of 8 K min-1 under N2 (20.0 dm3 h-1).
Synthesis of [Ni(tta)2(H2O)2] (3)
Complex 3 is accessible by the reaction of Ni(OAc)2.4H2O (111.99 mg, 0.45 mmol), dissolved in 50 ml hot ethanol and tta (199.971 mg, 0.9 mmol). The reaction was stirred in ethanol for 5 hours. Enough DW was added to precipitate the product and then washed several times with petroleum ether. The product dried under vacuum several days. light-green (Ni) solid was obtained in the yield of 85 %. Mp: 301-302 °C. IR (KBr), cm-1: 3431 (b, vs) (O-H); 3102 (m) (C-H); 1602 (vs) (CO); 1580 (s), 1540 (vs), 1507 (s) (C-C); 1458 (s), 1409 (vs), 1351 (s), 1308 (vs), 1258 (s), 1234 (s) (thienyl ring); 1191 (vs) (nC-F); 860 (s), 935 (s) (C-H out-plane thienyl); 788 (s), 726 (s) (nC-CF3). Anal. Calc. for C16H12F6O6S2Ni (536.9608 g/mol): C, 35.77 %; H, 2.25 %. Found: C, 36.10 %; H, 2.20 %. λmax (ε): 191 nm, 218 nm, 268 nm, 344 nm, 669 nm.
Synthesis of [Ni(tta)2(4,4’-bipy)]n (4)
Polymer 4 is accessible by the reaction of Ni(OAc)2.4H2O (111.99 mg, 0.45 mmol), dissolved in 50 ml hot ethanol with tta (199.971 mg, 0.9 mmol). After 5 hours of stirring at room temperature, (70.286 mg, 0.45 mmol) of 4,4´-bipyridine was added as one portion. A light yellow-brown precipitate was appeared after 20 minutes, and resulting solution was stirred continuously for another 5 hours at room temperature. The precipitate was filtered off, washed with chloroform, ethanol, water, and then dried under vacuum for several days to produces a light green solids. Yield of 95%. Mp: > 400 °C (no melting). IR (KBr), cm-1: 3447 (bs) (O-H); 3072 (m) (C-H); 1603 (vs) (CO); 1576 (s), 1539 (s), 1506 (vs) (C-C); 1470 (s), 1412 (vs), 1353 (s), 1303 (vs), 1251 (s), 1231 (s) (thienyl ring); 1189 (vs) (nC-F); 860 (s), 934 (s) (s) (C-H out-plane thienyl); 714 (s), 785 (s) (nC-CF3). Anal. Calc. for C26H16F6N2O4S2Ni (657.2634 g/mol): C, 47.56 %; H, 2.44 %; N, 4.27 %. Found: C, 47.35 %; H, 2.55 %; N, 4.25 %.
Synthesis of [Ni(tta)2(pz)]n (5)
Polymer 5 is prepared in an analogous manner to polymer 4. In this respect, Ni(OAc)2.4H2O (111.99 mg, 0.45 mmol) and pyrazine (36.04 mg, 0.45 mmol) were used producing a light green solids in a yield of 95 % (based on [Ni(tta)2(H2O)2] (3), 0.409 mmol). Mp: 351 °C. FT-IR (KBr), cm-1: 3068 (m) (C-H); 1605 (vs) (CO); 1580 (s), 1539 (vs), 1508 (s) (C-C); 1469 (s), 1413 (s), 1352 (s), 1299 (b, vs), 1255 (s), 1231 (s) (thienyl ring); 1188 (vs) (nC-F); 859 (s), 930 (s) (s) (C-H out-plane thienyl); 788 (s), 714 (s) (nC-CF3). Anal. Calc. for C20H16F6N2O4S2Ni (583.16.g/mol): C, 41.38 %; H, 2.07 %; N, 4.83 %. Found: C, 41.31 %; H, 2.17 %; N, 4.81 %.
Synthesis of [Ni(tta)2(1,4-dip)]n (6)
Polymer 6 is prepared in an analogous manner to complex 4. In this respect, Ni(OAc)2.4H2O (111.99 mg, 0.45 mmol) and 1,4-diisocyanobenzene (1,4-dip) (57.66 mg, 0.45 mmol) were used instead of Co(OAc)2.4H2O and 4,4´-Bipy, respectively to produce a light green solids. Yield of 95%. Mp: 290 °C (dec.). IR (KBr), cm-1: 1603 (vs) (CO); 1580 (vs) (C-C); 1540 (s), 1507 (s), 1458 (s), 1410 (s), 1351 (s) (thienyl ring); 1306 (vs), 1258 (s), (C-C and C-R); 1234 (s), 1192 (vs) (nC-F); 1131 (vs), 1149 (s) (C-H in-plane thienyl). Anal. Calc. for C24H16F6N2O4S2Ni (631.2 g/mol): C, 45.66 %; H, 2.56 %; N, 4.44 %. Found: C, 46.21 %; H, 2.43 %; N, 4.08 %.
Result and discussion
Synthesis and characterization
The reaction of 1-thenoyl-4,4,4-trifluoroacetone (H-tta: 1) with Ni(OAc)2.4H2O (OAc = O2CMe) in a 2:1 molar ratio gave [Ni(tta)2(H2O)2] (3: tta = deprotonated of 1) complex in ethanol, which was isolated green solid after precipitation upon treatment with aqua (Scheme 1). The produced complex is soluble with most common organic solvents including tetrahydrofuran, acetonitrile, and ethanol. However, in water and non-polar solvents 3 is not soluble. This complex is stable in both solution and solid state under the normal conditions. This stability may due to the presence of intermolecular forces between the mononuclear complex spheres of 3, as shown in the reported structures [14]. The gentle heating of the title complex solid, in an oven up to 180 ºC, change the solubility to be non soluble in various organic solvents. The poor solubility indicates for turning into the di- or polynuclear ones by oligomerization through the bridging oxygen atoms of diketonate unit as known and observed of such systems [22].
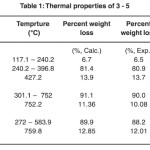 |
Table 1: Thermal properties of 3-5 Click here to View table |
Complex 3 can be extended to infinite coordination polymers of [Ni(tta)2(X)]n (X = 4,4′-bipy (4), pz (5), 1,4-dip (6)) by the reaction of nickel atom in 3 with s-donor bridging ligand such as 4,4-bipyridine (4,4′-bipy), pyrazin (pz), and 1,4-diisocyanobenzene (1,4-dip) in warm ethanol in a 1:1 molar ratio (Scheme 1). These polymers can also be prepared directly by stoichiometric reacting of Ni(OAc)2.4H2O (OAc = O2CMe) with 1 and s-donor ligand in 1:2:1 molar ratio, respectively for 6 hours of reaction stirring (Scheme 1). The aqua ligands in 3 are eliminated by a strong σ–donor bridging ligand forming 4-6, wherein the complex and polymeric structures were proposed according the reported of similar materials [13, 14, 16 – 21]. The solutions and solids of polymers are stable in air. After appropriate work-up, polymers 4 – 6 could be isolated as light green solid. They are none dissolving in most common organic solvents including tetrahydrofuran, acetonitrile, and ethanol. However, in water and non-polar solvents also are not soluble.
The elemental analyses of 3 – 6 agree with their formula as shown in Experimental section. The chemical nature is characterized by Ft-IR .
The reaction progress of 1 with 2 could additionally be controlled by IR spectroscopy, since the characteristic absorptions of the free non-coordinated b-diketone H-tta disappeared during the course of the reaction and new bands characteristic for metal b-diketonato species were observed (Experimental). IR spectrum of 3 shows prominent absorptions at 3431 cm-1 (ascertains the presence of coordinated aqua ligands [23]) and 1600 – 1410 cm-1 (typical for metal b-diketonate complexes [24 – 25]). IR spectroscopy can also be used to monitor the elimination of the aqua ligands in 3 by thermal treatment because the very characteristic vibrations of the aqua ligands continuously disappear with progress of the reaction. This could additionally be proven by thermogravimetric studies showing that 3 eliminate its two aqua ligands between 117 – 240 ºC (Figure 1) [14, 24]. The peaks at 1602, 1580, 1540, and 1507 are assigned to the keto-enol tautomerism chelating ring of νC=O and νC=C stretching vibrations [18]. The observed downfield shift, going from free ligand (1: νC=O = 1652 and νC=C = 1580 cm-1) to the corresponding vibrations in 3 indicates for the complexation, which found in consistent with the reported one [19]. The presence of these bands and their shifts (DnC=O) should be regarded as a characteristic stretching vibrations of keto-enol tautomerism chelating ring of tta ligands with Ni(II) centre as a whole, as in case of benzene [20]. The appearance of a vibration at 3102 cm-1 indicates the formation of hydrogen bonds in 3 [26], this can be considered as evidence for the formation of supramolecular complexes in solid state. The stretching vibration of νC-F for the coordinated tta (in 3) is found at 726 cm-1 (3). This vibration is shifted to a somewhat lower frequency (for comparison H-tta (1): 732, 746 cm-1). The stretching vibration of the C-H out-of-plane of thienyl ring at 803 cm-1 in 1 is shifted to the lower frequency upon complexation in 3, which is observed at 791 cm-1.
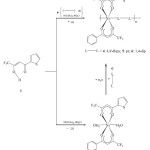 |
Scheme 1. Synthesis 3-6. Click here to View scheme |
 |
Figure 1 – 5 Click here to View figure |
The reaction progress of 3 with s-donor bridging ligand can be controlled by IR spectroscopy, since the characteristic absorptions of the coordinated aqua ligands in 3 [14] disappeared during the course of the reaction. This indicates the successful substituting the terminal aqua ligands in 3 by 4,4′-bipy, pz, 1,4-dip ligands forming 4 – 6. This substituting could be proven by thermogravimetric studies showing that 4 – 6 do not include aqua ligands between 110 – 143 ºC (Figure 1). As result of changing the aqua ligands in 3 with strong σ–donor bridging ligand such as 4´,4-bipyridine ligand, the stretching vibration of C-CF3 shifted to the lower frequency (714 cm-1) compared to 3 (726 cm-1). Furthermore, the stretching vibrations of C-H of thenoyl ring shifted to the lower frequencies (3072 cm-1) compared to 3 (3102 cm-1). This is ascribed to an increase in the σ–donating and in the back-donating form Ni(II) à tta. IR spectrum shows the prominent absorptions in the range of 1602 – 1412 cm-1 (typical for metal b-diketonate complexes [12, 14 – 27]).
The UV–Vis spectrum also confirmed the formation of Ni(tta)2(H2O)2 (3) (tta = deprotonated–1) complex, wherein the main absorption peaks are summarized in the Experimental section. The main absorption peaks in 1 are 274 and 322 nm. The first weak peak (λmax = 274 nm) is attributed to the π–π* absorption band in COketo form, while the second intense one (λmax = 322 nm) can be attributed to π–π* absorption band CO enol form [26] G. Gilli, V. Bertolasi, V. Ferretti and P. Gilli, J. Acta Crystallogr., B 49 (1993), pp. 564–576. Full Text via CrossRef[13, 28]. The intensity ratio of COketo toCO enol was 1.83. This is considered as evidence for the complex formation in enol-tta system. In addition, one intense peak at 193 nm and one shoulder peak at 216 nm are appeared. Upon coordinating 1 with nickel ion to form 3, the π–π* absorption band of CO enol form is red-shifted to around 344 nm. Furthermore, the π–π* absorption band of COketo form is red-shifted to around 268 nm. This red shift could be rationalized in term of raising HOMO energy level upon complexation. Moreover, the shoulder peak is appeared as intense band at 218 nm without any significance shift. With increasing the concentration of complex we saw one weak broad peak at λmax = 669 nm which correspond to the d–d transition.
Due to the poor solubility of 4 -6 polymers the UV-Vis spectroscopy cannot be measured.
Thermal properties
ThermoGravimetric (TG) studies were carried out with 3 – 5 to evaluate the decomposition behaviour in the range of 25 to 800 °C with increasing temperature in order to examine the thermal stability and to obtain 1st information on the decomposition temperature of 3 – 5 (see Table 1). Measurements were conducted at atmospheric pressure under a nitrogen purge (1.0 cm3 min-1) with a constant heating rate of 8 °C min-1.
Under typical TG conditions the decomposition of 3 starts at 117 °C and is completed at 750 °C as shown in Figure 1. The weight loss was accompanied by the residue in the TG pan, the amount of which is almost in accordance with the theoretical percentage calculated for the formation of nickel oxide. Between 117 – 240 ºC the observed weight loss is 6.5 % which corresponds to the eleimination of the two H2O ligands (6.7 %) producing [Ni(tta)2] (vide supra). Afterwards, the two tta ligands are eliminated between 240 – 700 ºC (observed weight loss, 80.9 %; calcd. 81.4 %). The residue remaining in the TG pan is nickel oxide as it could be shown by calculation (theoretical percentage of the formation of nickel oxide, 13.9 %; observed residual weight, 13.7 %).
The DSC curve of the 3 in a flowing N2 gas at a heating rate of 10 ◦C.min−1 is illustrated in Figure 2. There are three endothermic processes and one exothermic process. The peak temperature and enthalpy of endothermic processes are 203.641 ◦C (136.433 – 215.847 ◦C) and DH = 111.744 J g−1, 270.54 ◦C (257.602 – 294.034 ◦C) and DH = 59.811 J g−1, and 335.104 ◦C (319.005 – 352.163 ◦C) and DH = 28.445 J g−1. This indicates for three steps of changing in structure and decomposition, which is not clearly appeared in TGA. The first endothermic process is related to the weight loss of two coordinated water, while the second and the third endothermic process are related to the partial decomposition process or coordination sphere rearrangement.
Under typical TG conditions the decomposition of 4 starts at 301 °C and is completed at 752 °C as shown in Figure 3. The weight loss was accompanied by the residue in the TG pan, the amount of which is almost in accordance with the theoretical percentage calculated for the formation of nickel oxide. The disappearance of weight loss between 117 – 240 indicates for the successful displacement of 4,4’-bipy ligand with aqua molecules. The two tta and one 4,4’-bipy ligands are eliminated between 301 – 752 ºC in one step of decomposition (observed weight loss, 90.0 %; calcd. 91.1 %). The residue remaining in the TG pan is nickel oxide as it could be shown by calculation (theoretical percentage of the formation of nickel oxide, 11.36 %; observed residual weight, 10.08 %).
Under typical TG conditions the decomposition of 5 starts at 272 °C and is completed at 583 °C as shown in Figure 4. The weight loss was accompanied by the residue in the TG pan, the amount of which is almost in accordance with the theoretical percentage calculated for the formation of nickel oxide. The disappearance of weight loss between 117 – 240 °C indicates for the successful displacement of pz ligand with aqua molecules. The two tta and one pz ligands are eliminated between 301.1 – 752 ºC in one step of decomposition (observed weight loss, 88.2 %; calcd. 89.9 %). whereby this decomposition peak in DTG is appeared in two peaks one at 343 ºC (Derivative Weight percentage = – 9.648 % min-1) and another intense peak at 359 ºC (Derivative Weight percentage = – 19.426 % min-1). In the end of this decomposition step (at 759 ºC), nickel oxide could be formed (theoretical percentage of the formation of cobalt oxide, 12.85 %; observed residual weight, 12.01 %).
The DSC curve of the 5 in a flowing N2 gas at a heating rate of 10 ◦C.min−1 is illustrated in Figure 5. In the DSC curve of 5, there os one endothermic process. The peak temperature and enthalpy of endothermic process are 375.55 ◦C (331.94 – 387.539◦C) and DH = 196.473 J g−1. This indicates for one step of changing in structure and decomposition, which is clearly appeared in TGA. This endothermic process is related to the weight loss of two tta and pz ligands.
Conclusion
The mononuclear complex 3 has been successfully prepared and characterized. The produced complex is extended to infinite metal-organic coordination polymer of [Ni(tta)2(X)]n (X = 4,4′-bipy (4), pz (5), 1,4-dip (6)). Thermal properties proved a good stability for polymers. Complex 3 is formed in enol-tta system. The formation of hydrogen bonds can be considered as evidence for the formation of supramolecular complex of 3 in solid state. Thermal properties proved a good stability for polymers. Two enol-tfa systems were shown by electronic spectra through binding with metal atom.
References:
- a) Bourne S. A., Lu J. J., Mondal A., Moulton B. and Zaworotko M. J., Angew. Chem., Int. Ed. Engl., 40: 2011 (2001). b) Holiday B. J. and Mirkin C. A., Angew. Chem., Int. Ed. Engl., 40: 2022 (2001).
- M-C. Dul, E. Pardo, R. Lescouëzec, Y. Journaux, J. Ferrando-Soria, … R. Ruiz-García, J. Cano, M. Julve, F. Lloret and D. Cangussu, Coord. Chem. Rev., 254: 2281(2010).
- J. W. Steed and J. L. Atwood, Supramolecular Chemistry, (Wiley: USA). 2005
- G. R. Desiraju, Angew. Chem., Int. Ed. Engl. 34: 2311 (1995).
- T. Kuroda-Sowa, T. Horrino, M. Yamamoto, Y. Ohno, M. Maekawa and M. Munakata, Inorg. Chem., 36:6382 (1997).
- H.M. Daoud, M.Sc Thesis, supervised by Dr. Mohammed Ahmed Al-Anber, Mutah University, 2009.
- M.A. Al-Anber, Mahdi Lataifeh, Haneen Dawoud, J. Macromolecular Sci.: Part B, Physics, 52:344 (2013).
- D. Ma, Y. Wu and X. Zuo, Material letters, 59:3678 (2005).
- H. Gallardo, G. Conte, P. Tuzimoto, A. Bortoluzzi, R. A. Peralta and A. Neves, Inorg. Chem. Commun., 11:1292 (2008).
- P. A. Vigato, V. Peruzzo and S. Tamburini, Coord. Chem.Rev., 253:1099 (2009).
- H. O. Omoregie and J.A.O. Woods. Archives App. Sci. Res., 2(4): 7-16 (2010).
- M. A. Al-Anber, Int. J. Chem. Sci. Techno. 3(1):40 (2013).
- M. Al-Anber, H. Daoud, T. Ruffer and H. Lang, J. Mol. Struct., 997:1 (2011).
- M. Al-Anber, P. Ecorchard, T. Rüffer and H. Lang, Main group Chem., 11:205 (2012).
- M. A. Al-Anber, H. M. Daoud, T. Rüffer, H. Lang, Arabian J. Chem. In press (2012) DOI: 10.1016/j.arabjc.2012.04.048
- A. W. Maverick, F. R. Fronczek, E. F. Maverick, D. R. Billodeaux, Z. T. Cygan and R. A. Isovitsch, Inorg. Chem., 41:6488 (2002).
- J. Lu, C. Yu, T. Niu, T. Paliwala, G. Crisci, F. Somosa and A. J. Jacobson, Inorg. Chem., 37:4637 (1998).
- B-Q. Ma, S. Gao, T. Yi and G-X. Xu, Polyhedron, 20:1255 (2001).
- C. Chen, D. Xu, Y. Xu, C. Cheng, Acta Crystallogr., Sect. C, 48:1231 (1992).
- M.J. Plater, M.R. St. Foreman and A.M.Z. Slawin, Inorg. Chim. Acta 303:132 (2000).
- Y.Z. Xu and S.Shi, Acta Chim. Sini., 44:336 (1986).
- A. I. Matesanz, I. Cuadrado, C. Pastor and P. Souza, Zeitschrift für anorganische und allgemeine Chemie, 631:780 (2005).
- M.A. Hassaan Aly and M.G. Marei, J. Indian Chem. Soc., 70:64. (1993).
- K. Jeyasubramanian, S. Abdul Samath, S. Tambidurai, R. Murugesan and S. K. Ramalingam, Trans. Met. Chem., 20:76. (1995).
- J. Lu, C. Yu, T. Niu, T. Paliwala, G. Crisci, F. Somosa and A. J. Jacobson, Inorg. Chem., 37:4637 (1998).
- M. Indrani, R. Ramasubramanion, S. Kumaresan, S.K. Kang, M. Chen and M. Du, Polyhedron, 27:3593 (2008).
- M. A. Al-Anber, Int. J. Chem. Sci. Techno., 3(1):33 (2013).
- Gilli, V. Bertolasi, V. Ferretti and P. Gilli, J. Acta Crystallogr., B 49:564 (1993).

This work is licensed under a Creative Commons Attribution 4.0 International License.









