Oxidation of Benzaldehyde by Quinolinium Chloro Chromate in Presence of Ctab in Sulphuric Acid Medium
Kanchan Kumar Rai1, Ravi Kant Kannaujia1,KarunaRai2 and SurjeetSingh
1Department of chemistry, Pt. Vasudev Tiwari Girls Degree College ,Jhansi (U.P.) -284002 2Department of chemistry, S.R. Degree College , Orai , Jalaun (U.P.) 3Department of chemistry, Om Harihar Degree College, Hamirpur, India
DOI : http://dx.doi.org/10.13005/ojc/290330
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
The study of oxidation of organic compounds is of immense importance both from mechanistic and synthetic points of view. It has a bearing on the chemical processes of life also. Investigation of the kinetics and mechanism of redox reactions has attracted the attention of chemists world over and mechanisms of several reactions have been clearly delineated. The mechanism of a chemical reaction cannot be fully described without the determination of its rate. The kinetic study of a wide range of chemical processes is seen to be of essential importance, not only in pure research but increasingly in industrial research, development and, in some instances, in quality control and analysis as well. Kinetic methods have become an essential technique in photochemistry, enzyme chemistry, study of chemical catalysis etc.
KEYWORDS:Benzaldehydes;QCC;Thermodynamic Parameters;CTAB
Download this article as:| Copy the following to cite this article: Rai K. K, Kannaujia R. K, Rai K, Singh S. Oxidation of Benzaldehyde by Quinolinium Chloro Chromate in Presence of Ctab in Sulphuric Acid Medium. Orient J Chem 2013;29(3). doi : http://dx.doi.org/10.13005/ojc/290330 |
| Copy the following to cite this URL: Rai K. K, Kannaujia R. K, Rai K, Singh S. Oxidation of Benzaldehyde by Quinolinium Chloro Chromate in Presence of Ctab in Sulphuric Acid Medium. Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=321 |
Introduction
Electron-transfer processes in micellar systems can be considered as models to get insight into electron-transport occurring in biological phenomena. It has been established that arrangement of hydroxy groups in polyhydroxylic molecules affects the oxidation rate of chromium (VI). There have been only a limited number of studies of chromium (VI) redox reactions in surfactant media. Therefore we have made systematic studies of the oxidative degradation of surfactants by different oxidant metal-ions. Kinetic studies are expected to provide information on how the electron-transfer event is affected by hydrophobic and electrostatic interactions between the micelles.
Chromium compounds are widely used as very powerful oxidation agents[1] for several functional groups pyridinium chloro chromate (Corey’s reagent) a air stable yellow solid is the reagent of choice for many types of oxidation reaction.
In the last few years we have been interested in the study of solvent effects in electron transfer reaction[2] the mechanistic pathways of the oxidation of organic compounds including substituted benzaldehydes by various recently[3]. It was observed that either the formation of a complex from the reactants or it’s deem position to products were proposed as the rate determining step in the benzaldehydes were often carried out in solvent mixture containing water and protic, hydrogen bond donor solvents such as acetic acid, t-butanol. etc.
A Kinetic mechanistic oxidation methionine by quinolinium chloro chromate has been reparted[4]. Quinolinium chloro chromate is a mild and selective oxidant. Oxidation of the various compound[5]with QCC were reported. Kinetics and mechanism of oxidation of formic and oxalic acid aliphatic primary alcohols by quinolinium bromo chromate was reparted. Quinolinium dichromate oxidation of aliphatic aldehydes and benzaldehydes were reparted.
Oxidation of diphenacyl sulphide by quinolinium chloro chromate was studied in acetonitrile medium. The oxidation of 10, 20 and 30 alcohol by quinolinium fluoro chromate has been reported[6]. In the kinetic of reaction of completed Cr (VI) species by pyridinium and quinolinium chloro chromate have all ready reparted. Pyridinium chlorochromate acts as two electron oxidant in kinetic studies of the oxidation lactic acid and mandelic acid in acidic medium[7]. Pyridinium chloro chromate has been used as a mild and selective oxidizing reagent in synthetic organic chemistry.
Oxidation of α – hydroxy acids by other pyridinium and quinolinium halochromate have also been made. The catalytic effect of some bidentate ligands on the oxidation of lactic acid by Cr (VI) has also been reparted[8].
Effect of acetyl tri methyl ammonium bromide on the oxidation of tetra ethylene gycol by N-chlorosaccharin in acidic acid medium has been reported[9].
In the development of new chromium (VI) reagent for the effect and selective oxidation of compound under milled conditions some important entries are – pyridinium dichromate pyridinium in florochromade, pyridinium bromo chromate, Quinolinium chloro chromate, fluoro chromate, etc.
The use of quinolinium chloro chromate has an oxidant is well documented for the oxidation of primary and secondary alcoholorganic sulphidessubstituted benzaldehydes, benzyl alcohols[10], Aromatic anils , lactic and glycolic acid, methionine[11], D-fructose[12], D – Mannos, unsaturated organic substrate [13], acrylic acid, 2-Furaldehyde[14]and D-galactose etc.
Materials & Method
In the kinetic studies of Oxidation of benzaldehydes by QCC in presence of CTAB in acidic media, different chemicals were used in the form of solution. The procedure employed for the preparation of these solutions and the kinetics studies have been describes in the Method.
1- Standard Ferrous Ammonium Sulphate Solution:-
The stock solution of F A S (0.001 M) was prepared by dissolving known amount of ferrous ammonium sulphate. Some amount of sulphuric acid is added to it to stop the oxidation of solution.
Potassium Dichromate Solution:-
Similarly the stock solution of potassium dichromate (0.001 M) was also prepared by dissolving appropriate amount of substance. Both the solutions were standardized by usual methods in the presence of n- phenyl anthranilic acid.
These solutions were used to determine the rate of reaction by back titration method.
Sulphuric Acid Solution:-
Stock solution of sulphuric acid was prepared by diluting its appropriate volume with distilled water. The concentration of acid was determined by titrating it against standard alkali solution.
Indicator Solution
Stock solution of sulphuric acid (AR, Merk) was prepared by diluting its appropriate volume with distilled water. The concentration of acid was determined by titrating it against standard alkali solution with phenolphthalein indicator. 3-4 drops of indicator solution were used in each titration.
Preparation of Quinolinium Chloro Chromate:-
For preparing QCC 6 M chromium oxide solution needed the molecular weight of chromium oxide is 99.99 so to prepare its 250 ml. 6 m. Solution, 24.9975 gm. chromium oxide solid added to 250 ml. distilled water.
Preparation of Quinoline:-
It can be safely prepared from quinoline, chromium tri oxide and hydrochloric acid (1:1:1).
184 ml (1.1 M) of chromium oxide solution in hydrochloric acid is rapidly stirred. After 5 minutes the solution is cooled up to 0oC and quinoline was carefully added. After 10 minutes orange yellow colored precipitate were obtained which is filtered under vacuum. The precipitate washed and dried. The obtained QCC was stored in dark place.
Preparation of CTAB:-
Stock solution of cetyl trimethyl ammonium bromide (0.05 M)was prepared by dissolving accurately weighed amount of CTAB in doubly distilled water.
Kinetics Methods:
All the standard flask and reaction bottle were of borosilicate glass with well ground stoppers. The volumetrically apparatus, pipettes, burettes and flask were standardized by the usual methods. The glasswares were used, pretreated with chromic acid and then rinse with distilled water.
An electro pretreated thermostatic water bath used. As is usual, it was provided with sufficient thermal logging, suitable heaters and stirrers use.
Rate measurements
In rate measurement following procedure was adopted. The kinetics studies were made by taking excess amount of sucrose acid concentration over the oxidant concentration at constant ionic strength. The reaction mixture, containing appropriate quantity of oxidant solution, acids and water were taken in 100 ml conical flask. The substrate solution was taken in another flask. These two flasks were kept in a thermostat maintained at required temperature. The total volume of reaction mixture was 50 ml after allowing sufficient time for the reactants to attain the temperature of the water bath. They were mixed to each other .The progress of reaction was monitored by withdrawing aliquot at definite time intervals, in a another conical flask containing unreacted ferrous ammonium sulphate solution was titrated against potassium dichromate solution using n phenyl anthranilic acid as an indicator.
Kinetic Studies
Evolution of Kinetics results
The pseudo first order the rate constant expressed in min-1 were obtained in each oxidation form from the rate equation
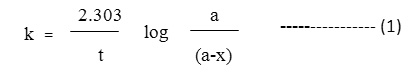
The second order rate constant expressed in L mol1 min-1 were obtained by dividing the first order rate constant with (substrates).
k2 = k1 / [substrates] —————– (2)
Typical Kinetic Runs:
In the oxidation studies for each substrate different kinetic runs shell be carried out to investigate the general behavior of the reaction under different experimental conditions. These studies are the effect of concentration of the oxidant substrate, sulphuric acid solvent, neutral salt and the variation of temperature.
Order of Reaction:
The order of reaction with respect to oxidant and substrate shell be determined by Ostwalled’s methods. Kinetics studies shell be carried out at varying concentration of oxidant keeping the other condition constant to obtained the order of reaction with respect to substrates, has been determined by the use of the given equation.
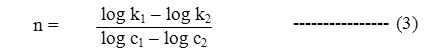
The value of (n) shows the order with respect to reactant whose concentration is varied.
Thermodynamic Parameters:
The thermodynamic parameters such as temperature coefficient, energy of activation, frequency factor, enthalpy of activation, entropy of activation and free energy of activation has been evaluated in the research work. The effect of temperature was studied at different temperature.
Temperature Coefficient:
Temperature has a profound effect on the reaction rate. The ratio of two values of two constant have been 100C difference known as temperature coefficient, is given by equation

Energy of Activation:
Arrhenius showed that the velocity constant (k) of a chemical process increases exponentially with temperature. He therefore suggested empirical relation such as,
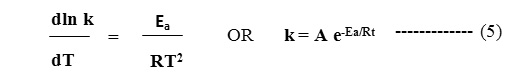
In this Arrhenius equation, k is the specifics reaction rate. A is preexponential or frequency factor. The term Ea is the activation energy, R is the universal gas constant and T represents the temperature in Kelvin. The standard method for obtaining Ea is either to evaluate from Arrhenius equation or from graph. Both the methods have been applied as detailed below
Arrhenius Equations
The activation energy can be calculated by appropriately filling the Arrhenius equation
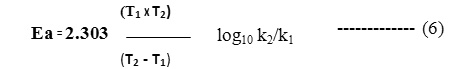
Graphical Methods:
The energy of activation was also obtained by plotting log k1 against 1/T. Arrhenius plot was found to be linear on for the data records. The energy of activation can be calculated by following equation:
Gradient = Ea /2.303 R
Ea = -slope x 2.303R ————-(7)
(c) Frequencies factor (A) which represents the total frequencies of the encounters between the two reactant molecules, irrespective of whether they possesses sufficient energy or not is given by
log A = log k1 + Ea /2.303RT ———– (8)
Heat of Reaction
Thermodynamically we have the following relation for heat of reaction
ΔH≠ = Ea – RT ———- (9)
Entropy of Activation:
Entropy of activation is evaluated by employing the equation
ΔS≠ = 2.303 R (log A – log e-kT/h) ————– (10)
Where, k = Boltzmann Constant = 1.38 X10-23 JK-1
T = Absolute Temperature
h = Plank Constant = 6.626 x10-34 J sec
e = 2.718, R = Universal, Gas constant = 8.3144 JK-1mor-1
Free Energy of Activation:
The change in free energy (ΔG≠), gives the extent to which a reaction goes to completion. It is determined from the following relationship
ΔG = ΔH≠– T ΔS ≠ ————— ( 11)
Where, ΔH≠ & ΔS≠ are the change in enthalpy and entropy and T is the absolute temperature. The calculated values of, all the activation parameter have been recorded.
Results And discussion
The kinetic results of the miceller effect on oxidation of benzaldehyde by QCC in sulphuric acid medium under studies are summarized and there behavior towards oxidation is discussed on the basis of the kinetic results.
Order of reaction:
All oxidation of benzaldehyde are homogeneous and obey with respect to kinetics. The order of reaction indicates the relationship between the concentration of reactants and the rate of reaction. It tells us the number and types of each molecule participating in the formation of the transition state of the rate determining step.
Dependence of Rate of Oxidation:
The rate constant for all the reaction is determined under conditions, where all reagents are maintained in large excess compared to the concentration of one of the reactant. By a series of such experiments in which the concentration of the species in excess are change systematically.
Dependence of Reaction Rate on Benzaldehyde concentration:
The rate of reaction increases with increase in the concentration of benzaldehyde.
Data from Tables 1 shows that the rate of reaction is linearly increased by increasing substrate concentration. The order of reaction was further verified by studying the reaction under pseudo first order conditions taking the different concentration of benzaldehyde keeping the QCC concentration constant.
The plot of k1 vs [Benzaldehyde] is a straight line passing through the origin, therefore by confirming that the order in respect of benzaldehyde is one. A plot of log k1 vs log [benzaldehyde] is a straight line with a slope of 1.15. It indicates that the reaction is first order with respect to [benzaldehyde]. Further the value of k2 to corresponding substrate concentration is constant i.e. k1 [benzaldehyde], this confirms the first order dependence in substrates concentration in both cases. In order to verify the first order behavior of this oxidation process a graph between 1/k1 vs 1/[s] was drawn an examination of the curve shows that 1/k1 is linearly related to 1/[s] and line passing through the origin in both curves, which directly
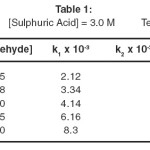 |
Table 1: Click here to View table |
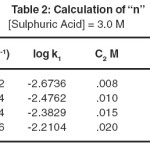 |
Table 2: Calculation of “n” Click here to View table |
shows the first order behavior of reaction and further suggested that no stable complex between QCC and substrates is formed.
Dependence of Reaction Rate on QCC concentration:
During the oxidation carried out in the presence of excess substrate with different initial concentration of QCC at constant temperature. The concentration of benzaldehyde and sulphuric acid were kept constant but the concentration of QCC varied. Table 3 records the results for different QCC concentrations. The reaction follows the first order kinetics with respect to disappearance of QCC. Concentration-time plot for individual run was found to be straight line. Thus the oxidation process is independent of QCC.
Temperature – 350C , Reaction Mixture – Over all Concentration
[Benzaldehyde] – 0.10 M, [Sulphuric Acid] – 3.0
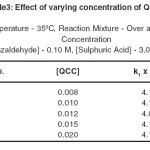 |
Table- 3 Effect of varying concentration of QCC Click here to View table |
3. Dependence of Reaction Rate on H2S04 Concentration:
It was found that without sufficient concentration of acid the oxidation process is very slow. In order to see the influence of acid concentration on oxidation of benzaldehyde by QCC the kinetics were followed at different concentrations of sulphuric acid, keeping that of others constant. The relevant kinetic data are shown in table 4. The values of rate constant increases with increase in sulphuric acid concentration, which signifies that the reaction is acid catalyzed. The plot of log k vs log [H+]2 was found linear and passing through the origin. This confirms the dependence of rate on the second power of concentration of sulphuric acid.
The linear plots with slope nearly 2, obtained by the k vs [H+]2 plot. This further suggests that the order of reaction is two in H2S04. Values of k2 are found constant in both cases.
![Table 4: Effect of varying [H2SO4]](http://www.orientjchem.org/wp-content/uploads/2013/11/Vol29_No3_kanchan_oxi_t4-150x150.jpg) |
Table 4: Effect of varying [H2SO4] Click here to View table |
On the basis of above discussion it is obvious that the order of reaction with substrate and oxidant is one while the order with respect to acid is two. Thus the rate expression should be of following form:
Rate = k1[V(v)] [substrate] [H2S04] 2 ———————-(1)
Dependence of Reaction Rate on CH3COOH Concentration:
The rate of reaction in solution is influenced by the solvent used as the reaction medium. Theoretical treatment of solvent effect on reaction rate has mostly taken the macroscopic dielectric constant to be the significant solvent variable.
The acid catalyzed oxidation of benzaldehyde was studied in solution containing different proportions of acetic acid – water. It was observed that increase in percentage of acetic acid in the solvent mixture, decreases the rate, suggesting that a medium of low dielectric constant favors the oxidation (table 5) .This observation points to either an ion-ion or cation – dipole type of interaction between the oxidant and the substrate. But change in ionic strength of the medium has marginal effect on rate.
![Table 5: Effect of varying [CH3COOH]](http://www.orientjchem.org/wp-content/uploads/2013/11/Vol29_No3_kanchan_oxi_t5-150x150.jpg) |
Table 5: Effect of varying [CH3COOH] Click here to View table |
Dependence of Reaction Rate on [(NH4)2S04]:
The ionic strength is very important to decide the nature of the species present in the rate controlling step. The initial rate in the presence of added ammonium sulphate was measured. Table 6 shows that there is no effect of ionic strength on rate of reaction.
![Table 6: Effect of varying on the [(NH4) 2 SO4]](http://www.orientjchem.org/wp-content/uploads/2013/11/Vol29_No3_kanchan_oxi_t6-150x150.jpg) |
Table 6: Effect of varying on the [(NH4) 2 SO4] Click here to View table |
Dependence of Reaction Rate on temperature
The addition of CTAB catalyzed the reaction. As the reported critical micellar concentrationof CTAB is 9.2 x 10-4 at 250C, the concentration of CTAB varied from 2.0 x 10-3 to 1.0 x 10-2 mol dm3 and rate of reaction increases as reported in table 7.
![Table 7: Effect of varying [CTAB]](http://www.orientjchem.org/wp-content/uploads/2013/11/Vol29_No3_kanchan_oxi_t7-150x150.jpg) |
Table 7: Effect of varying [CTAB] Click here to View table |
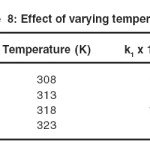 |
Table 8: Effect of varying temperature Click here to View table |
Micelles are formed due to assembling of amphiphilic molecules of surfactant above a certain concentration called critical micellar concentration . Micelle catalysis of reaction of in aqueous solution is usually explained on the basis of a distribution of reactants between water and the miceller ‘pseudo phase’. The miceller may provide a favorable orientation of the reactant by polarity gradients. In the present reaction, hydrophobic interaction is most likely to be operative due to relative larger hydrocarbon chains of the substrate[15-16]. This interactive localization of the reacting species in the relatively small volume of the micells compared to the bulk solution leads to a large increase in the effective concentration and as a result the observed rate increased accordingly. The other most probable reason seem to be electro static attraction between polar CTAB and the micelle.
Dependence of Reaction Rate on temperature.
Lastly it is useful to examine the Arrhenius parameter in oxidation studies interims of there reasonableness and consistency with the transition state theory. The reaction rate increased considerably on increasing the temperature in the range 350C to 500C or above. Rate constants for benzaldehyde at different temperature have already been presented in activation parameters obtained from the Eyring’s rate theory at 350 C are summarized
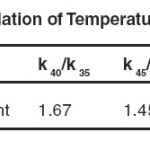 |
Table 9: Calculation of Temperature coefficient Click here to View table |
It is linear plot is obtained by plotting log k1 vs 103/T, in each case obeying the Arrhenius law. The nearly same energy of activation of the order of 74 k j mol-1 suggests probably the similar type of mechanism is operative in the oxidation in the presence and absence of CTAB.
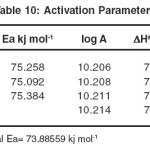 |
Table 10: Activation Parameters Click here to View table |
Average Ea = 74.242 kj mol-1 , Graphical Ea = 73.88559 kj mol-1
It is seen that the energy of activation for these reactions is characteristic of a bimolecular reaction and entropy of activation in each case is a negative value. This suggests that the intermediate formed in each case has a more rigid structure than the reactants.
CONCLUSION:
The influence of organized media (micelles. vesicles, membranes, etc.) on the course of a large variety of chemical and biochemical reactions is now well documented. Ionic micelles typically increase rates of reactions of reactive counter ions with hydrophobic substrates that bind to micelles. These rate increases are due to higher local concentrations of both reactants at the micelle- water interface as corn pared to their stoichiometric concentrations. Surfactants are referred as amphiphilic, amphipathic, heteropolar or polar/nonpolar compounds as they possess distinct regions of hydrophobic (water repelling) and hydrophilic (water loving) character in their molecules. The utilization of surfactants as reaction media affects rates, products and in some other cases, stereochemistry of the reactions. Mechanistic work on micellar effects is typically done using reactant concentrations much below those required for normal preparative work. Micellar solutions in some cases have proved superior to organic solvents as reaction media to obtain better yield. Evidence is now accumulating which indicate that studies of chemical reactions in micellar media could provide an understanding about the reactions, which take place at the interface.
REFERENCES:
- S.V. Ley, A. Madin, Oxidation Adjacent to Oxygen of Alcohols by Chromium Reagents in Comprehensive Organic Synthesis, Programon Press, Oxford, 1991.
- G. Fatima, Jeyanthi, K.P. Elango, Int. J. Chem., Kinet, 2003, 35, 154.
- K. Mahanti, K.K. Banerji, J. Indian Chemical Soc. 2002, 29, 31.
- M. Randeeswaram, B. Johns, D.S. Bhuvaneshwari and K.P. Elango, J.Serb Chem. Soc. 2005, 70(2) 145-151.
- S. Saravanakanna, K.P. Elango, Int, H. Chem. Kinet, 2002, 34, 585.
- Meenakshi Sundram and R. Sockalingam, collect. Czeck Chem, Common 2001, 66, 877-896
- S. Jain, B.L. Hiran and C.V. Bhatt, E.Journal of Chem, 2009 ,6(1), 273-280
- B.L. Hiran and G. Chaturvedi, J. Indian Chem. Soc. 2004, 84, 556
- Vandana Sharma, K.V. Sharma and V.W. Bhagwat, E. Journal of Chem. 2008, 5(3), 598-606.
- B. Özgün and N. Değirmenbaşi, Monatsh. Chem. 135 (2004) 483
- M. Pandeeswara, B. John, D. S. Bhuvaneshwari and K. P. Elango, J. Serb. Chem. Soc. 70 (2005) 1
- J. V. Singh, K. Mishra and A. Pandey, Oxid. Commun. 26 (2003) 235
- K. Mishra, J. V. Singh and A. Pandey, Bull. Polish Acad. Sci. 51 (2003) 25
- G. S. Chaubey, B. Kharsyntiew and M. K. Mahanti, J. Phys. Org. Chem. 17 (2003) 83
- Fisher L R and Oakenful D G, Quart .Rev. Chem.Soc,. 1977, 6, 25
- BacchaJM,Kaul A K, Prasad B and Ramegowda N S,Indian J. Chem., 1973, 11, 609

This work is licensed under a Creative Commons Attribution 4.0 International License.









