Mg/Triethylammonium formate: A useful system for reductive dimerization of araldehydes into pinacols; nitroarenes into azoarenes and azoarenes into Hydrazoarenes
M Geeta Pamar1, P Govender1, K Muthusamy1, Rui W. M. Krause2 & H M Nanjundaswamy1,2,3*
1School of Life sciences, Westville Campus, University of KwaZulu-Natal, Durban, 4000 South Africa 2Department of Chemistry, Rhodes University, PO Box 94, Grahamstown, 6140, South Africa. 3Regional Assistant Chemical Examiner’s Laboratory, Department of Health services, Hampanakatta, Mangalore-575 001 India
DOI : http://dx.doi.org/10.13005/ojc/290316
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
Studies are reported which describes the effectiveness of triethylammonium formate in presence of magnesium for the efficient intermolecular pinacol coupling using MeOH as solvent. Various aromatic carbonyls underwent smooth reductive coupling to give the corresponding 1,2-diols. A series of azo compounds were obtained by the reductive coupling of nitroaromatics while azo compounds were reduced to the corresponding hydrazoarenes by this system. There was no adverse effect on the other reducible and hydrogenolysable groups such as ether linkage, hydroxy and halogens. The reactions are clean, high yielding and inexpensive. All the reactions proceeded smoothly at ambient temperature.
KEYWORDS:Triethylammonium formate;magnesium;pinacol coupling;reduction;azo compounds
Download this article as:| Copy the following to cite this article: Pamar M. G, Govender P, Muthusamy K, Krause R. W. M, Nanjundaswamy H. M. Mg/Triethylammonium formate: A useful system for reductive dimerization of araldehydes into pinacols; nitroarenes into azoarenes and azoarenes into Hydrazoarenes. Orient J Chem 2013;29(3). doi : http://dx.doi.org/10.13005/ojc/290316 |
| Copy the following to cite this URL: Pamar M. G, Govender P, Muthusamy K, Krause R. W. M, Nanjundaswamy H. M. Mg/Triethylammonium formate: A useful system for reductive dimerization of araldehydes into pinacols; nitroarenes into azoarenes and azoarenes into Hydrazoarenes. Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=288 |
Introduction
Reduction of organic compounds is important synthetically both in the laboratory and in industry. In comparison with catalytic reduction using molecular hydrogen, transfer reduction using hydrogen donors has real and potential advantages1,2. Molecular hydrogen, a gas of low molecular weight and therefore high diffusibility, is easily ignited and presents considerable hazards, particularly on the large scale; the use of hydrogen donors obviates these difficulties in that no gas containment is necessary, no pressure vessels are needed, and simple stirring of solutions is usually all that is required. Potentially, transfer methods could afford enhanced selectivity in reduction.
In the presence of hydrogenation catalysts, certain organic compounds can serve as the hydrogen source. The best hydrogen donors for heterogeneous catalytic transfer hydrogenation comprise of simple molecules such as cyclohexene3, 1,4-cyclohexadiene4, hydrazine5, formic acid6 and formates7, phosphinic acid8 sodium tetrahydroborate9 sodium phosphinates10 and indoline11. The efficiency of alkylammonium formates as a hydrogen donor in the palladium-catalyzed reductive dimerization of conjugated dienes has been studied by Roffia et. al12.
Triethylammonium formate is successfully used for the reductive dehalogenation of aromatic halides and nitro compoundsin presence of Pd/C and Pt/C respectively13. It is also worth to note that triethylammonium formate is known for the reduction of mono and dinitro compounds, α, b-unsaturated carbonyl compounds and acetylenes in presence of palladium14. Later the same system was utilized for the reduction of acetylenes to cis-monoenes and hydrogenolysis of tertiary allylic amines15. Recently, D. C. Gowda et. al have reported the facile synthesis of azo compounds from aromatic nitro compounds using ammonium acetate catalyzed by pb16.
Results and discussion
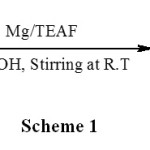
Very recently we found that hydrazinium sulfate in presence of magnesium for the rapid generation of arylhydroxylamines in water17. We also have used hydrazinium formate as an efficient reagent for the selective protection of carbonyl compounds as azines without any catalyst18.The effectiveness of both hydrazinium sulfate, hydrazinium formate and the utility of triethylammonium formate described above, prompted us to examine triethylammonium formate for possible organic transformation. Also to continue the research programme in developing methods for organic transformations employing inexpensive metals in combination with easily available reagents19, we thought it would be useful to investigate the reduction of carbonyl compounds in presence of zinc and to better define the usefulness of triethylammonium formate, however we observed the pinacolization of carbonyl compounds as shown in Scheme 1. Pinacolization of benzaldehyde 1a, an aromatic aldehyde was studied initially as a representative example to optimize this reaction.
 |
Scheme 1 Click here to View figure |
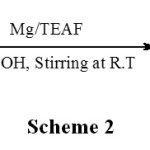
To a vigorously stirred suspension of 1.5 equivalent triethylammonium formate, freshly distilled benzaldehyde (1.06 g, 10 mmol) in methanol (5 mL) was added followed by commercial magnesium turnings (1.1 eq) and stirring was continued at room temperature to recover the corresponding pinacol. 1H NMR (CDCl3): d 2.33 (s, 2H, OH), 4.78 (s, 2H, PhCH-), 7.20–7.27 (m, 10H, Ar); m/z (%): 214 (1.6), 180 (2.8), 167 (2.0), 108 (88.00), 107 (100.00) 79 (82.40), 77 (48.00); IR (KBr): cm-1 3300–3529. This result prompted us to check other substituted aromatic aldehydes and ketones for the suitability of the reaction and found that benzaldehyde, substituted benzaldehydes furfuraldehyde and acetophenones to give the corresponding 1,2-diols. The substituents to benzaldehyde such as CH3, OCH3, Cl, and Br were unaffected and gave the desired products. The reactions of substituted araldehydes by the present method have been found to be sensitive to the electronic and steric effects of the substituents. The electron donating (inductive and mesomeric) groups seem to decrease the extent of conversion. Further, an attempt for pinacolization of hindered ketones such as benzophenone and substituted benzophenones with this system failed and were reduced to the corresponding benzhydrols as shown in Scheme 2 and the results are presented in table 1 (entries 3a-c). Treatment of diaryl ketones with this system for 1.5-2 h afforded the corresponding benzhydrols with 40% yield. When this reaction was carried out in the presence of 2 equiv. of triethylammonium formate with 2 equiv. of Zn, the yield improved to 55−62%.
 |
Scheme 2 Click here to View figure |
 |
Table 1. Rapid pinacolization stirring at 25 ºC using Mg/TEAF-MeOH Click here to View table |
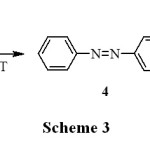
It is interesting to note that, when nitrobenzene is subjected for the above reaction condition, we could observe the rapid change in colour from colourless to orange colour and analyses revealed the formation of azobenzene with a trace amount of hydrazobenzene as shown in Scheme 3 and the method was extended to the reductive coupling of various substituted nitrobenzenes to the corresponding azoarenes and the reduction of azoarenes to the respective hydrazoarenes as shown in Table 2 and Table 3.
 |
Scheme 3: Click here to View figure |
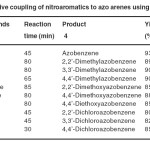 |
Table 2. Reductive coupling of nitroaromatics to azo arenes using Mg/TEAF-MeOH Click here to View table |
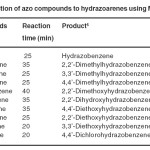 |
Table 3. Reduction of azo compounds to hydrazoarenes using Mg/TEAF-MeOH Click here to View table |
Experimental Section
All carbonyl compounds and substituted nitrobenzenes were of commercial grade; commercially available reagents and solvents were used throughout and purified before use. Yields refer to the isolated products after purification. Analytical TLC performed on precoated aluminum plates with Merck silica gel 60 F-254 as the adsorbent. The developed plates were air dried and irradiated with UV light. GC analyses were performed on a SHIMADZU GC-MS QP 5050A instrument. IR spectra were recorded on NICOLET 400D FT-IR Spectrometer. The 1H NMR spectra were recorded on Bruker 300 MHz spectrometer as CDCl3 solutions with TMS as internal standard.
General procedure for the rapid pinacolization
To a vigorously stirred suspension of triethylammonium formate (15 mmol), aromatic aldehyde/arylmethylketone (10 mmol) in aqueous methanol (5 mL) was added followed by magnesium turnings (10 mmol). After the completion of the reaction, the reaction mixture was filtered and the residue was washed with water, ethyl acetate (2 ´ 10 ml). The filtrate was diluted with water (25 ml) and repeatedly extracted with ethyl acetate (3 ´ 15 ml). The combined organic extracts and washings were washed with water (2 ´ 10 ml), dried over anhydrous sodium sulfate. Solvent removal under reduced pressure gave a white solid, which was further purified by recrystallization using dichloromethane and light petroleum (40-60°C) to get pinacol as white solid.
Typical procedure for the reduction of benzophenone
To a stirred solution of triethylammonium formate (15 mmol), magnesium turnings (12 mmol) was added the benzophenone (10 mmol) in methanol (5 mL) at room temperature and the resulting mixture was stirred for the appropriate time( Table 1). After the complete disappearance of the starting material, the resulting mixture was filtered and washed with diethyl ether. The filtrate was treated with saturated aqueous sodium hydrogen carbonate. The filtrate was extracted with ethyl acetate. Evaporation of the solvent followed by treatment of the residue with anhydrous sodium sulfate at room temperature afforded the benzhydrol.
Typical procedure for the reductive dimerization of nitrobenzene into azobenzene
To a solution of triethylammonium formate (15 mmol), taken in a round bottomed flask, nitrobenzene (10 mmol) in methanol (15mL) followed by magnesium turnings (12 mmol) were added and stirred at room temperature (25 ºC). After the completion of the reaction (monitored by TLC), the reaction mixture was filtered through celite pad, washed with diethyl ether. The filtrate was washed successively with saturated brine solution, water, dried over anhydrous sodium sulfate. Solvent removal and silica gel column chromatography gave azoarene.
Typical procedure for the reduction of azobenzene into hydrazobenzene
A mixture of azobenzene (182 mg, 1 mmol), triethylammonium formate (15 mmol), magnesium turnings (10 mg atom) and methanol (5 mL) in a round bottomed flask was stirred at room temperature (25 ºC). After the complete disappearance of the color of the starting material (orange to colourless), the reaction mixture was filtered through a Celite pad and washed with little diethyl ether. The combined filtrates and washings were evaporated under in vacuuo. The residue was purified either by preparative TLC or column chromatography to get hydrazobenzene (0.175 g, 95 %).
Conclusion
In summary, we have utilized triethylammonium formate for the effective preparation of pinacols which have wide range of utility. For example, a) 1,2-diols are useful synthons for a variety of organic synthesis22 b) constitutes an important method for the formation of carbon-carbon bonds23 c) frequently have been utilized as auxillaries in asymmetric synthesis24 d) in the synthesis of HIV-protease inhibitors and some natural products such as taxol25. This system is extended to the reduction of diaryl ketones to the corresponding benzhydrols, reductive dimerization of nitroaromatics to the corresponding azoarenes and azoarenes into hydrazoarenes in high yields. The advantages of this method are readily available and easily accessible reagents, works at ambient temperature which avoids the formation of olefin in case of formation of pinacols, reaction completed in shorter durations compared to the previous procedures and non requirement of inert atmosphere and ultrasonic conditions.
References
- a) Brieger G. and Nestrick T. J., Chem. Rev., 74, 567 (1974); Gladiali S. and Albereco E., Chem. Soc. Rev., 35, 226 (2006); (c) Wang C. Wu X. F. and Xiao J. L., Chem. Asian J., 3, 1750 (2008).
- Fahrenholtz K. E., J. Org. Chem., 37, 2204 (1972).
- a) Nishiguchi T. Tagawa T. and Fukuzumi K. J., Jpn. Oil Chem. Soc., 28, 174 (1979); (b) Nishiguchi T. Imai H. Hirase Y. and Fukuzumi K., J. Catal. 41, 249 (1976); (c) Kumari N. S. S. Khan S. A. and Sivanandaiah K. M., Indian J. Chem. 152 (1979); (d) Entwistle I. D., Tetrahedron Lett., 555 (1979); (e) Khan S. A. and Sivanandaiah K. M., Synthesis, 750 (1978) (f) Olah G. A. and Prakash G. K. S., Synthesis, 397 (1978) (g) Entwistle I. D. Johnstone R. A. W. and Telford R. P. J., Chem. Res (S)., 117 (1977); (h) Jackson A. E. and Johnstone R. A. W., Synthesis, 685 (1975); (i) Johstone R. A. W. Povall T. J. and Entwistle I. D., J. Chem. Soc., Perkin trans., 1, 1424 (1975); (j) Hanessian S. Liak T. I. and Vanasse B., Synthesis, 396 (1981); (k) Viswanatha V and Hruby V. J., J. Org. Chem., 45, 2010 (1980).
- a) Felix A. M. Heimer E. P. Lambros T. J. Tzougraki C. and Meienhofer J., J. Org. Chem., 43, 4194 (1978); (b) Rao V. S. and Perlin A. S., Carboh dr. Res., 83, 175 (1980); (c) Rao V. S. and Perlin A. S., Can. J. Chem., 61,652 (1983).
- a) Ayyanger N. R. Lugade A. G. Nikrad P. V. and Sharma V. K., Synthesis, 640 (1981); (b) Miyata T. Ishino Y. and Hirashima T., Synthesis, 834 (1978); (c) Anwer M. K. Khan S. A. and Sivanandaiah K. M., Synthesis, 751 (1978); (d) Entwistle I. D. Hussey B. J. and Johnstone R. A. W., Tetrahedron Lett., 4747 (1980).
- a) Nishiguchi T. Tagawa T and Fukuzumi K. J., Jpn. Oil Chem .Soc., 28, 174 (1979); (b) Nishiguchi T. Imai H. Hirase Y. and Fukuzumi K., J. Catal. 41, 249 (1976); (c) Entwistle I. D. Jackson A. E. Johnstone R. A. W. and Telford R. P., J. Chem. Soc. Perkin Trans. 1., 443 (1977) ; (e) Rao V. S. and Perlin A. S., Carboh dr. Res., 83, 175 (1980); (f) Rao V. S. and Perlin A. S., Can. J. Chem., 61,652 (1983).
- a) Entwistle I. D. Jackson A. E. Johnstone R. A. W. and Telford R. P. J. Chem. Soc. Perkin Trans. 1., 443 (1977); (b) Weir J. R. Patel B. A. and Heck F. R., J. Org. Chem., 45, 4926 (1980); (c) Bamfield P. and Quan P. M., Synthesis, 537 (1978); (d) Anwer M. K. and Spatola A. F., Synthesis, 929 (1980).
- a) Entwistle I. D. Jackson A. E. Johnstone R. A. W. and Telford R. P., J. Chem. Soc. Perkin Trans.1., 443 (1977); (b) Entwistle I. D. Johnstone R. A. W. and Telford R. P. J. Chem. Res (S)., 117 (1977).
- Nishiguchi T. Tagawa T. and Fukuzumi K. J., Jpn. Oil Chem. Soc., 28, 174 (1979).
- a) Entwistle I. D. Jackson A. E. Johnstone R. A. W. and Telford R. P., J. Chem. Soc. Perkin Trans.1., 443 (1977); (b) Johnstone R. A. W. and Wilby A. H., Tetrahedron, 37, 3667 (1981); (c) Entwistle I. D., Tetrahedron Lett., 555 (1979); (d) Entwistle I. D. Hussey, B. J. and Johnstone R. A. W., Tetrahedron Lett., 4747 (1980).
- a) Nishiguchi T. Tagawa T. and Fukuzumi K. J., Jpn. Oil Chem. Soc., 28, 174 (1979); (b) Nishiguchi T. Imai H. Hirase Y. and Fukuzumi K., J. Catal., 41. 249 (1976).
- Roffia P. Gregorio G. Conti F. and Pregaglia G. F. J., Organomet. Chem., 55, 405 (1973).
- Cortese N. A. and Heck R. F., J.Org. Chem., 42, 3491 (1977).
- Cortese, N. A. and Heck, R. F., J.Org. Chem., 43, 3985 (1978).
- Weir J. R. Patel B. A. and Heck R. F., J.Org. Chem., 45, 4926 (1980).
- Srinivasa G. R. Abiraj K. and Channe Gowda D. C., Synth Commun.,33, 4221 (2003).
- Pasha M. A. and Nanjundaswamy H. M., Org. Chem. an Indian J, 2, 5-6 (2006).
- Nanjundaswamy H. M. and Pasha M. A., Synth. Commun., 36, 3161 (2006).
- a) Pasha M. A. and Myint Y. Y., Ultrason Sonochem., 13, 175 (2006); b) Pasha M. A. and Rizwana S., Indian J. Chem., 44B, 420 (2005); (c) Nagaraja D. and Pasha M. A., Indian J. Chem., 41B, 1747 (2002); (d) Rama K. Nagendra E. and Pasha M. A., Indian J. Chem., 39B, 563 (2000).
- Ian H. and Bunbury H M., Dictionary of Organic Compounds, 5th Edn, Chapman & Hall, London, 1982.
- Vogel, A. I., Text Book of Practical Organic Chemistry, 3rd Edn, Longman Group Limited, London, 1968.
- Nicolaou K. C. Yang Z. and Liu, J. J., Nature, 367, 630 (1994).
- a) McMurry J. E., Chem Rev, 89, 1513 (1989); (b) Wirth, T., Angew Chem Int Ed Engl, 35, 61 (1996).
- Schmidt B. and Seebach, D., Angew Chem Int Ed Engl, 30, 99 (1991).
- a) Kammermeier B. Beck G. Jacobi D. and Jendralla H., Angew. Chem. Int. Ed. Engl., 33, 685 (1994); (b) Shiina I. Nishimura T. Ohkawa N. Sakoh H. Nishimura K. Saitoh K. and Mukaiyama T., Chem Lett., 419 (1997); (c) Nicolaou K. C. Yang Z. Liu J. J. Ueno H. Nantermet P. G. Guy R. K. Clyborne C. F. Renaud J. Couladouros E. A. Paulvannan K. and Sorensen E. J., J. Am. Chem. Soc., 117, 634 (1995); (d) Nazare M. and Waldmann H., Angew. Chem. Int. Ed. Engl., 39, 1125 (2000); (e) Kim S. M. Byun I. S. Kim Y. H., Angew. Chem. Int. Ed. Engl., 39, 728 (2000).

This work is licensed under a Creative Commons Attribution 4.0 International License.









