Fractal Aggregation of Copper Particles using Electroless Cell
S.Q.Chishty1, Mazahar Farooqui2 , Mohd. Khizer3, B.B Rajeshaikh4
1Dept. of physics, Dr. Rafiq Zakaria College for woman Aurangabad. 2Dept. of chemistry, Dr. Rafiq Zakaria College for woman Aurangabad. 3Dept.of physics, Kohinoor college of arts science and commerce Khultabad. 4Dept. of physics, Govt. College of arts science Aurangabad.
DOI : http://dx.doi.org/10.13005/ojc/290348
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
A phenomenon In which the particles performing Brownian motion when hit the aggregates become the part of it is known as Diffusion limited aggregation (DLA), which produces a fractal shape. Experimental efforts are discussed through which some DLA shapes arc obtained. For this purpose different electrolytic solutions are used. Electro less cells are also designed and constructed using standard methods. The cells have to be flexible in the sense that changing of plates and solutions should be easier for photography. We used compounds of copper,. for growth of fractals. Within a very short time metallic dendrites appeared in the cell at different operating conditions. These images were photographed, while desired branching structures in copper sulphate solution were seen. Results thus obtained are compared with the growth of DLA.
KEYWORDS:Electro-less cell;Fractal shapes;DLA
Download this article as:| Copy the following to cite this article: Chishty S. Q, Farooqui M, Khizer M, Rajeshaikh B. B. Fractal Aggregation of Copper Particles using Electroless Cell. Orient J Chem 2013;29(3). doi : http://dx.doi.org/10.13005/ojc/290348 |
| Copy the following to cite this URL: Chishty S. Q, Farooqui M, Khizer M, Rajeshaikh B. B. Fractal Aggregation of Copper Particles using Electroless Cell. Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=358 |
Introduction:
In many fractal growth experiments structures are more complicated. The most common experiments are Electro-deposition with point electrode at center and circular electrode around it. When suitable electrolytic solution is poured in and proper potential difference is maintained between the electrodes, a self-similar and complicated metallic deposition develops at the center. This type of model is known as diffusion limited aggregation model (DLA) In above experiments aggregation or identical particles takes place to cluster through a Brownian motion
1. The study of DLA provides a basis for understanding a large range of natural pattern and formation phenomena.
2. Here we used a simple cell, known as electro-less cell to study DLA.
Design of Cell
We used an Aluminum plate or area 6045 cm2 that is sandwiched between two glass plates. On the surface of Aluminum plate a paper of same size is kept and is called as dielectric spacer [3]. On this paper an electrolytic solution (copper sulphate etc.) is poured and spread in proper quantity. Since the porous paper acts a semi permeable membrane. It should have more
rag contents and should not be of a glazed surface. A plastic coat or smooth surface papers are therefore avoided. Paper used for cyclostyling is preferred. Function of upper glass plate is to apply equal pressure and also to check the faster evaporation of solution. The whole arrangement is leveled one and is kept on a table.
Effect of Concentration Electrolytic Solutions:
In this cell various electrolytic solutions of the compounds of nickel, zinc, copper, silver, lead, and manganese were tried. For some solutions we obtained metallic deposition but copper sulphate has shown good results [4]. Concentration of solution played an important role in the deposition process and affected the shape of the growth pattern. It was observed that slight change in the concentration created a considerable change in the growth pattern. If concentration of the solution is increased then, ions concentration, also increases, as a result deposition becomes crowded and the structure becomes more compact and tends towards non-fractals. If concentration is decreased the growth become similar to DLA [5] with clearly defined branches. Low concentrations results in a slow and unclear deposits which does not have continuity and shapes are not well defined. In the present work the growth were studied using copper sulphate solutions of concentration ranging from 0.5 M to 2M in the steps of 0.5 M. Effect of concentration can be clearly seen in the set of three photographs in figure 1.
Time Factor:
Deposition mainly depends upon the time. With the increase in the time more particles tend to diffuse towards the aggregates and becomes the part of the cluster. The process starts after nearly an hour when different dark points are observed on the surface of white paper. Cluster goes on increases with time, branches slowly begins to appear on the surface white paper. For full growth pattern experiment takes nearly 24 to 48 hours. At the initial stage the growth is comparatively faster and becomes slow at the later stages. There are several clusters found on the white paper [5]. When copper deposits get oxidized, looses their shapes and characteristics, it is the decaying process. After nearly 96 hours the deposition gets automatically pasted on the surface of a white paper and one can slowly remove the upper and lower glass plates to preserve the structure.
Effect of Porous Spacer:
During the experimental work, various kinds of porous spacers of different thick nesses were used. We started from filter paper of thickness 0.01 cm , thick paper (bond papers) of thickness 0.023 cm, cyclostyling papers (more porous) of thickness 0.013cm. The cotton mixed cloths of thickness 0.027 cm was also tried. We successfully obtained desired shapes of deposits when cyclostyling paper or cloths are used as porous media, while filter papers give very poor results as they do not support the solution to a predetermined time. If the thickness of the spacers is increased beyond 0.1 cm , then there are no deposits. Therefore, thickness of porous spacers is
much important and considered as one of the parameters which controls the process. Strength of paper quality is also important; therefore only white cyclostyling papers are used in the cells.
Chemical Process Governing the Deposits:
When copper sulphate solution is spread over white papers in the above described cell chemical process starts after few minutes. Since copper is eoectropositive with redox potential is + 0 . 3 3 7 V and aluminum is electronegative with redox potential -1.66V so there is an exchange of aluminum ions with copper ions in copper sulphate solution. Copper gets deposited on aluminum plates and aluminum ions are released and a copper bridge is formed connecting aluminum p late with copper sulphate solution. There is formation of a nucleation center on the surface of a membrane [6]. The charge exchange between the copper ions and aluminum plate on the membrane surface takes place. Thus initiating the process of Diffusion Limited Aggregation [7]
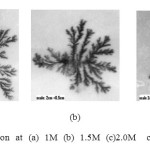 |
Figure l: Copper deposition at (a) 1M (b) 1.5M (c)2.0M concentration of CuSO4 Click here to View figure |
Photography
To obtain images of the fractal shapes grown on white papers a single lens reflecting (SLR) camera was used with zoom and close up lens kit attachments . The size of the growth patterns were small to include finer details of the growth making zoom and close up lenses became essential. All the photo- graphs are developed and printed in laboratory keeping the records. These prints are scanned using 300dpi scanner, these images digitized and converted into matrix In a bit map file (BMP) [8).We developed different computer programs In turbo basic for the analysis of shapes. using formula
D = lim(log N )/Log (r)
Fractals dimensions 0 were computed and a graph of box size (Log r) versus number of boxes (Log N)[9) is plotted as shown in figure2.
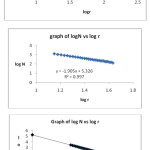 |
Figure 2: Box counting for shapes shown In figure dimensions are (a) 1.88 (b) 1.90 (c) 1.76 Click here to View figure |
Results & Discussion:
It is observed that the copper sulphate solution in the cell produces better deposits. Thickness of spacers or semi-permeable membrane also plays an important role in the development of shapes. Concentration of solution is an important factor for which one has to be very careful. At higher concentrations the growth may deviate from the true DLA and at very low concentrations ion responses become weaker and weaker.
Use of properly developed computer Programme to reduced the time consuming task of characterizing the shapes obtained in the cell. Repeated trials have to be carried out to reduce statistical variations arising from the starting point . At 1 M concentration of the solution the fractal dimensions are closer to DLA. At relatively higher concentrations 1.5M and 2M branching becomes crowded and patterns lends to deviate more from true DLA structure.
References
- B B Mandelbrot ‘The Fractal Geometry of Nature’ W.H. Freeman and Co. New York (1982).
- Vicsek; T.A. ‘Fractal Growth Pheno.nena’world scientific Singapore (1992).
- Paranjpc: A.S. l.alwani: S.K; Josh, V.M. Ind. J.purc and App. Phy. (1997) 35, 316-321.
- Peitgen, H.O., Jurgens, 1-1., Saupe, D., ‘Chaos and Fractals New Frontiers of Science’, Springer -Verlag, New York (1992)
- Hasting; H.M. Sugihara G. Fractal A user’sguide for the Natural Sciences’ oxford university press (1995).
- R.E. Amritkar; Indian Journal of pure & app. Phy. (1994). 32595-601
- Witten T.A and Sander, L.M. phy rev. lett. (1981) 47, 1400.1407
- Markus Alber and Joachim Peinke phy. rev. lett. (1998) 57.5489-5493
- J.S. Tarafdar and S .Roy ;Fractal,3, No.1 (1995),99-103

This work is licensed under a Creative Commons Attribution 4.0 International License.









