Degradation of Phenol in Water by Titanium Dioxide Photocatalysis
Noureddine Barka1*, Idriss Bakas2, Samir Qourzal2, Ali Assabbane2, Yhya Ait-Ichou2
1 Université Hassan 1, Laboratoire CAE, Equipe de Recherche Gestion de l’Eau et Développement Durable (GEDD), Faculté Polydisciplinaire de Khouribga, BP. 145 Khouribga, Morocco. 2 Equipe de Matériaux, Photocatalyse et Environnement, Département de Chimie, Faculté des Sciences, Université Ibn Zohr, B. P. 8106 Cité Dakhla, Agadir, Morocco
DOI : http://dx.doi.org/10.13005/ojc/290328
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
The photocatalytic degradation of phenol in a static-batch photoreactor, using a UV lamp as a light source and TiO2 as a photocatalyst, was investigated. The effects of various parameters, such as photocatalyst dosage, initial substrate concentration, reaction pH and addition of oxidants, on the phenol degradation rate of TiO2 photocatalysis were examined. The degradation rates proved to be strongly influenced by these parameters. Adsorption of phenol is a prerequisite for the TiO2-assisted photodegradation. In the presence of both UV light illumination and TiO2 catalyst, phenol was more effectively degraded than with UV alone. Phenol showed higher photocatalytic oxidation efficiency at natural pH. The reaction rate was found to obey pseudo first-order kinetics represented by the Langmuir-Hinshelwood model. A complete photomineralization of phenol can be easily achieved.
KEYWORDS:Advanced Oxidation Processes;Photocatalysis;TiO2; Photomineralization;Phenol;Water treatment
Download this article as:| Copy the following to cite this article: Barka N, Bakas I, Qourzal S, Assabbane A, Ait-Ichou Y. Degradation of Phenol in Water by Titanium Dioxide Photocatalysis. Orient J Chem 2013;29(3). doi : http://dx.doi.org/10.13005/ojc/290328 |
| Copy the following to cite this URL: Barka N, Bakas I, Qourzal S, Assabbane A, Ait-Ichou Y. Degradation of Phenol in Water by Titanium Dioxide Photocatalysis. Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=317 |
Introduction
A wide variety of organic pollutants are introduced into the water system from various sources, such as industrial effluents, agricultural run-off and chemical spills1. Their toxicity, stability to natural decomposition and persistence in the environment has been the cause of much concern to the societies and regulation authorities around the world.
Phenol is a kind of refractory organic compound, having the toxicity of carcinogenesis, teratogenesis and mutagenicity2. The treatment of phenol in micro-polluted water has always been paid attention by many researchers.
The traditional purification technique has been not suitable for the existing water source and water quality standards. Therefore, it is necessary to develop low cost and high efficient treatment technique.
Several specific new technologies, called Advanced Oxidation Processes (AOP), have been developed to eliminate dangerous chemicals such as organics from polluted waters. The photocatalytic process, based on UV irradiated semiconductor, represents one of AOP that provide an interesting route to the destruction of many organic substances to CO2, H2O and corresponding mineral acids3-5.
Semiconductor such as titanium dioxide TiO2-based photocatalytic oxidation has received much attention in environmental remediation field, especially for mineralization of many toxic and non-biodegradable organic pollutants in wastewater6,7. Among the photocatalysts, TiO2 (in anatase phase) has been most widely used because it is easily available, inexpensive, non-toxic, and shows a relative high chemical stability.
The process of the photocatalytic degradation of organic pollutants by the semiconductor powders has been considered to be very promising. Under UV light illumination, absorption of photons creates an electron-hole pair if the energy is higher than the bandgap (3.2 eV) of TiO2. The pairs migrate at the surface, are trapped by the titanium and OH surface groups, and finally from OH° and HO2° radicals. These free radicals cause the oxidation of organic compounds.
The aim of the present work is: (i) to investigate the influence of various parameters on photocatalytic degradation of phenol in the presence of TiO2 particles and irradiated by the UV light and (ii) to find out the optimum conditions for efficient degradation.
Experimental
Materials
All chemicals were reagent grade and were used without further treatment. Phenol was purchased from Fluka (> 99% purity). The photocatalyst was TiO2 “Degussa P-25” mainly anatase (80% anatase and 20% rutile) with a surface area of 50 m2/g, a density of 3.85 g/cm3 and an average particle size of 30 nm. The other chemicals used in this study such as K2S2O8 and H2O2 were obtained from Merck.
Photoreactor and light source
The static-batch photoreactor was a cylindrical flask of ca. 2 l, opened to air and fitted with a bottom optical Pyrex window ca. 4 cm in diameter (Figure 1). It was mounted at a distance of 3 cm from the top of the lamp assembly, which consisted of an HPK 125 W mercury lamp supplied by Phillips and a circulating-water cuvette (ca. 2.2 cm thick). A filter (Corning 0-52) was used to cut off the wavelengths shorter than 340 nm which can cause a direct photochemical transformation of phenol.
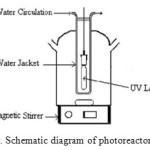 |
Figure 1. Schematic diagram of photoreactor system. Click here to View figure |
Procedure
In the experiments, 1000 ml of phenol at natural pH (6) was magnetically stirred in presence of TiO2 in the dark during 30 min to reach the adsorption equilibrium before UV irradiation. The suspensions were continuously purged with oxygen throughout in each experiment. The temperature during the experiment was maintained at 20°C. Samples of 0.5 ml were collected at regular intervals, filtered trough 0.45 nylon filters (Millipore) and analyzed by HPLC.
Analyses
The quantitative and qualitative analysis of the organic compounds in the samples was performed by High Performance Liquid Chromatography HPLC (Jasco-type). The wavelength of detector was 270 nm. A reverse-phase column (length, 25 cm; internal diameter, 4.6 mm) ODS-2 Spherisorb (Chrompack) was used. The mobile phase was composed of acetonitrile (20%) and doubly distilled water (80%). The flow rate was 0.4 ml/min.
Complete mineralization of organic samples to CO2 is very often obtained by using the photocatalytic method. The kinetic of the evolution of CO2 formed was followed by using the method of Chemseddine and Boehm8, which consists of flushing the CO2 produced by oxygen into a flask containing 500 ml of barium hydroxide (1.2 x 10-2 mol/l) and to follow the conductivity of the solution with a conductimeter Orion model 150. CO2 precipitates as BaCO3, thus decreasing the ionic conductivity in water.
Results and discussion
Kinetics of phenol disappearance
The elimination of phenol was studied at two different experimental conditions: (i) under UV illumination in absence of TiO2 (photolysis), and (ii) under UV illumination in presence of TiO2 (photocatalysis). In Figure 2, the normalized concentration of phenol is plotted as a function of reaction time. There was no observable loss of phenol when the irradiation was carried out in the absence of TiO2. However, in the presence of TiO2, a rapid degradation of phenol occurred by irradiation. The phenol concentration was completely removed after 120 min. Therefore, it can be concluded that phenol degradation in photocatalysis proceed by the oxidation of phenol by hydroxyl radical.
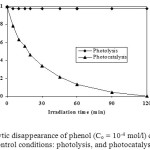 |
Figure 2. Photocatalytic disappearance of phenol (Co = 10-4 mol/l) degradation under the control conditions: photolysis, and photocatalysis. Click here to View figure |
Effect of photocatalyst dosages
To optimize the TiO2 suspension concentration, the effect of photocatalyst dosages on the degradation of phenol in aqueous solution was studied. The results were illustrated in Figure 3. The degradation efficiency increased with increasing the amounts up to 1g/l. Then, the efficiency decreased slightly, and above 1.25 g/l of TiO2 became nearly flat. The increase in the efficiency seems to be due to the increase in the total surface area, namely number of active sites, available for the
Photocatalytic reaction as the dosage of photocatalyst increased. However, when TiO2 was overdosed, the number of active sites on the TiO2 surface may become almost constant because of the decreased light penetration, the increased light scattering and the loss in surface area occasioned by agglomeration (particle-particles interactions) at high solid concentration9. Therefore, 1 g/l of TiO2 was selected as the optimal amounts of photocatlyst for the sequential experiment.
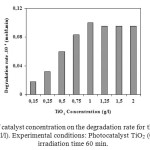 |
Figure 3. Influence of catalyst concentration on the degradation rate for the decomposition of phenol (Co = 10-4 mol/l). Experimental conditions: Photocatalyst TiO2 (0.15 g/l – 2 g/l), and irradiation time 60 min. Click here to View figure |
Effect of initial phenol concentration
Figure 4 presents the degradation of phenol, in solutions containing different concentrations of the starting compound and 1 g/l of TiO2 at its natural pH (6), as a function of the illumination time. The elimination of phenol proceeds in a shorter time period at lower concentrations of the pollutant, as expected.
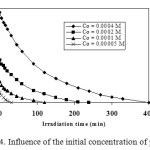 |
Figure 4. Influence of the initial concentration of phenol. Click here to View figure |
In Figure 5, it is shown that the initial degradation rate (ro) increases at the beginning of the run, when the concentration of phenol is increased until it attains a plateau, at around 1 .10-4 mol/l. This behavior indicates saturation-type Langmuir kinetics. This is confirmed by the linear plots of 1/ro versus 1/Co (Figure 6) with an intercept on the ordinate, in agreement with Eqs. (1) and (2):

hence
![]()
where Co is the initial phenol concentration. The factor k is often interpreted in the literature10 as a pseudo-first-order Langmuir-Hinshelwood (L-H)-type rate coefficient relating to TiO2-catalysed primary oxidation events on a surface monolayer (0.15 .10-4 mol/l.min) and K is a pseudo-equilibrium constant related to the monolayer adsorption (1.03 .104 l/mol). Such a Langmuir-type relationship between the initial degradation rate and the concentration, has been also reported by other authors11,12. This type of reaction kinetics suggests that the adsorption plays a key role in the photocatalytic degradation mechanism.
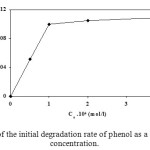 |
Figure 5. Variation of the initial degradation rate of phenol as a function of its initial concentration. Click here to View figure |
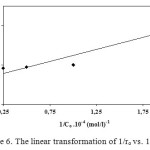 |
Figure 6. The linear transformation of 1/ro vs. 1/Co. Click here to View figure |
Effect of pH
An important parameter in the photocatalytic reactions taking place on the particulate surfaces is the pH of the solution, since it dictates the surface charge properties of the photocatalyst and size of aggregates it forms. Therefore, the degradation of the pollutant was studied at different pH values (in the range 2-10). The pH of the reaction mixture was adjusted by adding a dilute aqueous solution of HNO3 or NaOH. The degradation rate for the decomposition of phenol as a function of reaction pH is shown in Figure7.
The degradation efficiency increased with increase in pH. The zero point charge (zpc) pHzpc of TiO2 particles is around 6 13. TiO2 surface is positively charged in acidic media (pH < 6) whereas it is negatively charged under alkaline condition (pH > 6). Generally, the pH changes can have a non-insignificant result not only on the mode of adsorption of the phenol substrate on TiO2 surface, but also on the selectivity of the photodegradative reaction occurring on the particle surface redox reactions are very sensitive to changes in the surface potential. On the other hand, at high initial pH, more hydroxide ions (OH–) in the solution induced the generation of hydroxyl free radicals (OH°), which came from the photooxidation of OH– by holes forming on the titanium dioxide surface. Since hydroxyl free radical is the dominant oxidizing species in the photocatalytic process, the photocatalytic decay of phenol may be accelerated in an alkaline medium. Similar reaction has been suggested by a number of researches14,15. Consequently, pH 6 was selected for the optimal experimental conditions, because of the unnecessary of chemical treatment including neutralization process.
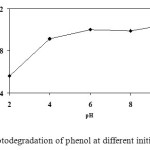 |
Figure 7. Photodegradation of phenol at different initial pH values. Click here to View figure |
Effect of electron acceptors
Since hydroxyl radical appears to play an important role in photocatalysis, electron acceptors such as hydrogen peroxide and potassium persulphate were added into the solution in order to enhance the formation of hydroxyl radicals and also to inhibit (e–/h+) pair recombination. The degradation rate for the decomposition of phenol in the presence of various electron acceptors is shown in Figure 8. All the additives showed a beneficial effect on the degradation of the model compound. One practical problem in using TiO2 as a photocatalyst is the undesired electron/hole recombination, which, in the absence of proper electron acceptor or donor, is extremely efficient and thus represents the major energy-wasting step thereby limiting the achievable quantum yield. One strategy to inhibit electron-hole pair recombination is to add other (irreversible) electron acceptors to the reaction. In highly toxic wastewater where the degradation of organic pollutants is the major concern, the addition of additives to enhance the degradation rate may often be justified. In this connection, we have studied the effect of electron acceptors such as H2O2 and K2S2O8 on the photocatalytic degradation of the model compound under investigation. These acceptors are known to generate reactive species according to the following Eqs. (3-5):
H2O2 + e– → OH° + OH– (3)
S2O82- + e– → SO42- + SO4°– (4)
SO42- + H2O → SO42- + OH° + H+ (5)
As expected, all the additives markedly influence the degradation rate for the decomposition of the compound. These results indicate that all the additives are more effective electron acceptors than molecular potential oxygen as indicated by the one electron reduction potential of different species formed from these additives: E(O2/O2°-) = -155 mV, E(H2O2/OH°) = 800 mV, E(S2O82-/SO4°-) = 1100 mV16. From the thermodynamic point of view all employed additives should therefore be more efficient electron acceptors than molecular oxygen.
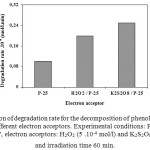 |
Figure 8. Comparison of degradation rate for the decomposition of phenol (Co = 10-4 mol/l) in the presence of different electron acceptors. Experimental conditions: Photocatalyst TiO2 ″Degussa P-25″, electron acceptors: H2O2 (5 .10-4 mol/l) and K2S2O8 (5 .10-4 mol/l), and irradiation time 60 min. Click here to View figure |
Photomineralization
The evolution of CO2 as a function of irradiation time in the photocatalytic degradation of phenol is shown in Figure 9. The same reactivity order was found for the total mineralization followed by CO2 evolution. Taking into account the fact that complete disappearance of phenol in the irradiated reactor occurs after 120 min, whereas the stoichiometric (equation 12) formation of CO2 is observed after 240 min under the same working conditions. This implies that total degradation requires a longer time for degrading all the possible reaction intermediate produced involved.
C6H5OH + 7O2 → 6CO2 +3H2O (6)
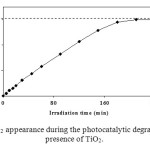 |
Figure 9. Kinetic of CO2 appearance during the photocatalytic degradation of phenol in the presence of TiO2. Click here to View figure |
Conclusion
The results of this study show that the TiO2 can efficiently catalyze the decomposition of phenol in the presence of light and oxygen. The photooxidation of phenol followed first-order kinetics. The apparent rate constant depends on the initial phenol concentration. The parameters such as concentration of the catalyst and pH play an important role affecting the reaction rate. The addition of electron acceptor markedly enhances the degradation rate of the pollutant. Complete photomineralization of phenol was achieved. The final degradation product was carbon dioxide, and all carbon atoms of phenol were transformed to CO2.
The observations of these investigations clearly demonstrate the importance of choosing the optimum degradation parameters to obtain high degradation rate, which is essential for any practical application of photocatalytic oxidation processes.
References
- Singhm H.K. and Muneer M., Research on Chemical Intermediates 30, 317 (2004.)
- Matos J., Laine J. and Herrmann J., Appl. Catal. B: Environ., 18, 281 (1998).
- Ziegmann M. and Frimmel F.H., Water Sc. Technolo., 61, 273 (2010).
- Barka N., Qourzal S., Assabbane A., Nounah A. and Ait-Ichou Y., Chem. Eng. com., 198, 1233 (2011).
- Çatalkaya E.Ç., Bali U. and Sengül F., Environ. Sc. Pollut. Res., 10, 113 (2003).
- Barka N., Qourzal S., Assabbane A., Nounah A. and Ait-Ichou Y., Arab. J. Chem., 3, 279 (2010).
- Abaamrane A., Qourzal S., Barka N, Mançour Billah S., Assabbane A. and Ait-Ichou Y., Orient. J. Chem., 28 (3), 1091 (2012).
- Chemseddine A. and Boehm H.P., J. Molecul. Catal., 60, 295 (1990)
- Qourzal S., Barka N., Belmouden M., Abaamrane A., Alahiane S., El Ouardi M., Assabbane A. and Ait-Ichou Y., Fres. Environ. Bull., 21, 1972 (2012).
- Barka N., Qourzal S., Assabbane A., Nounah A. and Y. Aît Ichou, J. Photochem. Photobiol. A: Chem., 195, 346 (2008).
- Terzian R. and Serpone N., J. Photochem. Photobiol. A: Chem., 89, 163 (1995).
- Qourzal S., Tamimi M., Assabbane A. and Y. Ait-Ichou, J. Colloid Interf. Sc., 286, 621 (2005).
- Parra S., Stanca S.E., Guasaquillo I. and Thampi K.R., Appl. Catal. B: Environ., 51, 107 (2004).
- Barka N., Assabbane A., Nounah A. and Aît Ichou Y., J. Hazard. Mater., 152, 1054 (2008).
- Piscopo A., Robert D. Weber and J.V., Appl. Catal. B: Environ., 35, 117 (2001).
- Faisal M., Tariq M.A. and Muneer M., Dyes and Pigments, 72, 233 (2007).

This work is licensed under a Creative Commons Attribution 4.0 International License.









