Complexation of Ciprofloxacin with Mn (Ii) Ion –A Potentiometric Study
Namita Bhardwaj
Department of Chemistry, Dr. C.V. Raman University, Kota, Bilaspur (C.G.), India.
DOI : http://dx.doi.org/10.13005/ojc/290347
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
The present study comprises determination of stability constant and the thermodynamics of interaction of transition metal ion Mn(II) with antibiotics Ciprofloxacin. The formation of 1:1 and 1:2 metal ligand complexes are found to be formed in 50 %( v/v) methanol – water system. The stability constant values for metal-ligand system compared at three temperetures, it is found that value of stability constant decreases with increasing temperature. This is in agreement with the conclusion of Pitzer1.
KEYWORDS:complexation;ligand;stability constant
Download this article as:| Copy the following to cite this article: Bhardwaj N. Complexation of Ciprofloxacin with Mn (Ii) Ion –A Potentiometric Study. Orient J Chem 2013;29(3). doi : http://dx.doi.org/10.13005/ojc/290347 |
| Copy the following to cite this URL: Bhardwaj N. Complexation of Ciprofloxacin with Mn (Ii) Ion –A Potentiometric Study. Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=356 |
INTRODUCTION
A Co-ordination compound (complex) is formed between a metal ion and molecules or ions called ligand. Transition metal complexes are cationic, anionic or neutral species in which transition metal is coordinated by ligands. Transition metals exhibit different oxidation states and can interact with a number of inorganic or organic molecules or ions (negatively or occasionally a positive ion) functions as ligand2.
Here in present study ciprafloxacin which belongs fluoroquinolones group of antibiotics is used as a ligand for complexation.The chemistry of metal-drug coordination compounds is more popular now than before particularly in the design of more biologically active drugs. [3] Metal ions are known to affect the action of many drugs. The efficacy of the drugs on coordination with a metal are enhanced in many cases.[4]Metal ions play a vital role in a vast number of widely differing biological processes and depending on their concentration, they may either contribute towards the health of the organism or cause toxicity.[5]
MATERIALS AND METHODS
A digital Systronics pH meter and a combination electrode (pH range 0-14) with an accuracy of 0.01 pH unit were used for the measurement of pH. In the proposed work chromatographically pure sample of antibiotics used (obtained from Fluka). For each titration fresh samples of antibiotics weighed and solution prepared to avoid the possibility of hydrolysis and photochemical decomposition.
The metal ion solution prepared from the corresponding nitrate of AR grade standardized by titration with disodium salt of EDTA as described by Schwarzenbach [6]. Carbonate free sodium hydroxide prepared by the method of Schwarzenbach and Biedermann and standardized by titration with pure oxalic acid. The modified form of Irving-Rosotti titration technique used [7].
Potentiometric titration carried out at three temperatures 250C, 300 C, and 350 C, keeping ionic strength of the solution constant (0.1 M KNO3). Presaturated N2 gas passed through the experimental solution before titration. During the titration the change in pH of the solution measured by a digital pH –meter provided with an electrode previously calibrated standard method.
The pKa values of ligand and stability constants have determined as the method described by Bjerrum [8],Calvin and Wilson [9] Fronaeous[10] Schwarzenbach [11] and Irving and Rossoti.
RESULT AND DISCUSSION
The stability constant values for complexation of ciprafloxacin with Mn(II) by Potentiometric titration method have been presented in table I and the thermodynamic parameters have been given in table II. The formation of 1:1 and 1:2 metal ligand complexes are found to be formed in 50 %( v/v) methanol – water system. The stability constant values for metal-ligand system compared at three temperatures, it is found that value of stability constant decreases with increasing temperature. This is in agreement with the conclusion of Pitzer that higher temperatures are not favorable for complex formation.
All the thermodynamic parameters calculated are found to be negative. The negative value of ΔG indicates that the complex formation is spontaneous. The negative value of ΔH indicates that reaction is exothermic. The negative value of ΔS indicates higher order in the complex formation.
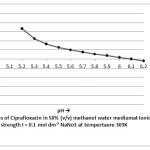 |
Figure 1 Click here to View figure |
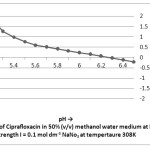 |
Figure 2 Click here to View figure |
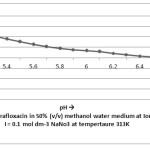 |
Figure 3 Click here to View figure |
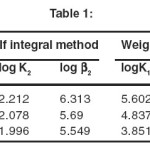 |
Table 1: Click here to View table |
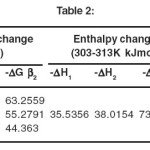 |
Table 2: Click here to View table |
References:
- K. S. Pitzer, J.Am. Chem. Soc. , 59, 2356 (1937)
- Co-ordination chemistry Vol.- 1,A.E. Martei Ed Von NostrandReinold Company, London (1971), 2A.
- G.Mendoza Diaj, R.Perez Alanso, R. Moreno Esparza, J, Inorg. Biochem 64, 207 (1996)
- J.A.Obaleye, J.B. Nde-aga, E.A. Balogun, Afr.J. Sci, 1, 10-12 (1997).
- S. Yambe, J.P. Koho, Chem. Abs. 111, 219307z (1989).
- H.M. Irving and H.S. Rossotti, J. Chem . Soc. , 2904(1956)
- Schwarzenback G., Flaschka H and Irving H.M. , Complexometric titration (Methuen,London)(1969)
- J.Bjerrum, ‘Metal Amine Formation in Aqueous Solution’. P. Hasse and Son, Copenhagen (1941)
- M.Calvin and K. Wilson, J. Am.Chem.Soc. 67, 2003(1945)
- S.Franaeous, Complex System hos Kopper , Gleerupska Universities-Bokhandeln.Lund (1948).
- G. Schwarzenbach and H. Ackermann, Helv. Chem. Acta, 30, 1798 (1947)

This work is licensed under a Creative Commons Attribution 4.0 International License.









