(4+2) Cycloaddition Reactions of 9-Substituted Anthracene Compounds
Adel AL-Saeedi1 and Mazahar Farooqui1,2*
1Post Graduate and Research Center, Maulana Azad College, Aurangabad (MS) India. 2Dr Rafiq Zakaria College for women, Aurangabad (MS) India
DOI : http://dx.doi.org/10.13005/ojc/290325
Article Received on :
Article Accepted on :
Article Published : 28 Oct 2013
The (4+2) cycloaddition or the Diels-Alder reaction is one of the most important reactions in the field of organic synthesis. The Diels-Alder reaction well known to all chemists is 100 years old. The Diels-Alder reaction during that period it has been studied intensively, where there are more papers, many books and reviews in the Diels-Alder reactions making the complete study of these works impossible, therefore in this article we concentrate on the review and generalization of the Diels-Alder reaction of 9-substituted anthracene with a variety of dienophiles.
KEYWORDS:Diels–Alder reaction;Synthesis;dienophiles;stereoselectivity;9-substituted anthracenes
Download this article as:| Copy the following to cite this article: AL-Saeedi A, Farooqui M. (4+2) Cycloaddition Reactions of 9-Substituted Anthracene Compounds. Orient J Chem 2013;29(3). doi : http://dx.doi.org/10.13005/ojc/290325 |
| Copy the following to cite this URL: AL-Saeedi A, Farooqui M. (4+2) Cycloaddition Reactions of 9-Substituted Anthracene Compounds. Orient J Chem 2013;29(3). Available from: http://www.orientjchem.org/?p=309 |
Introduction
Anthracene is a tricyclic aromatic hydrocarbon, it absorbs long wave UV radiation and it was first isolated in 1833 from coal tar. Since its discovery anthracenes have been found to have a large amount of utility across several subfields of chemistry.[1] Anthracene and its derivatives are among the most useful. In view of their fluorescent properties they are of practical interest as sensors and markers in biological or supramolecular systems. Their unique spectroscopic properties, in particular, have been studied in-depth.[2] as dyes for the study of lipid monolayers,[3] in organic light emitting diodes,[4] and in LCD displays.[5,6] Analogs have also been used to study the binding effects of ligands in DNA folding,[7]. anthracene are efficient photochromic system They dimer upon irradiation in a [4+4] cycloaddition and the dimer reverts back to its monomers on photolysis at lower wavelength or on thermolysis. Furthermore It was later determined that the precipitate was the dimer by calculation of its molecular weight (Scheme 1).[8]
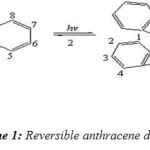 |
Scheme 1: Reversible anthracene dimerization Click here to View figure |
However, perhaps one of the more intriguing aspects of the chemistry of anthracene is the ability to undergo cycloaddition reactions with a variety of dienophiles across the C-9 and/or C-10 positions, presumably due to the ready availability of these compounds.
Diels-Alder Reactions
History of Diels-Alder Reactions
The (4+2) cycloaddition or Diels-Alder reactionis one of the most important C-C bond forming reactions in organic chemistry to form cyclic structures and is one of the major synthetic strategies employed to generate bicyclic compounds.[9-12] The reaction is named after Professor Otto Diels and his student Kurt Alder, two German chemists who studied the synthetic and theoretical aspects of this reaction in great detail. The Diels-Alder reaction well known to all chemists is 100 years old, but credit for its discovery can be traced to 1928 when the German chemists Otto Diels and Kurt Alder published the seminal article “Synthesen in der hydroaromatischen Reihe” in the journal Annalen der Chemie. The reaction studied by Diels and Alder is depicted in (Scheme 2). The importance of researches of Otto Diels and Kurt Alder was awarded the Nobel Prize in 1950. Diels-Alder reaction, the most powerful of synthetic reactions, presents a convenient and highly stereospecific route to the ubiquitous six-membered ring.[13-17] The Diels-Alder reaction has been studied intensively and it is under study almost in all leading world chemical centers.[18-27] Therefore has been published many books, [28-37] more papers and reviews dedicated in this reaction, these papers, reviews and books have further extended our knowledge in this field.[1, 38-43]
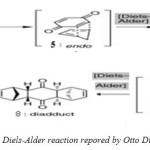 |
(Scheme 2: The Diels-Alder reaction repored by Otto Diels and Kurt Alder) Click here to View figure |
Diels-Alder reaction mechanisms
Though the Diels-Alder reaction was discovered in 1928 but the reaction mechanism is still in much controversy. There are three possible mechanisms which can account for most of the experimental data that have been accumulated for the Diels-Alder reaction. Currently the most favored mechanism is a concerted one in which there is a six-centered transition state with simultaneous formation of the two α bonds. However, there are two variations in the concerted mechanism (Scheme. 3). mechanism (1) a has a symmetrical transition state with α bond formation to the same extent, mechanism (2) has an unsymmetrical transition state with α bond formation to different extents resulting in localized partial charges on the diene and dienophile, mechanism (3) has a stepwise diradical transition state in which the diradical would be spin paired.[44]
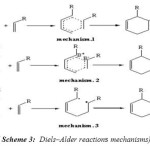 |
( Scheme 3: Diels–Alder reactions mechanisms) Click here to View figure |
Additions to 9-substituted anthracene
The Diels-Alder reaction between 9-substituted anthracene and monosubstituted dienophiles can yield there has been significant interest in the regioselectivity of these reactions. The first repoorted cycloaddition reactions of 9-substituted anthracene in this area cycloaddition reactions of 9-anthraldehyde with maleic anhydride, acrylic acid, acrylonitrile and allyl alcohol to assess its reactivity and selectivity with these dienophiles. In all cases the reactions proceeded to give predominately the ortho regioisomer (except maleic anhydride) (Scheme 4).[45]
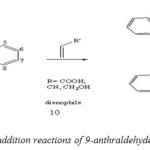 |
(Scheme 4: cycloaddition reactions of 9-anthraldehyde with dienophiles) Click here to View figure |
In these studies the ortho isomer is in agreement with theoretical observations concerning increased molecular orbital overlap with substituted dienes and dienophiles, and experimental observations for the Diels–Alder reactions of other substituted dienes and dienophiles.[46]
Diels-Alder reaction of 9-Cyanoanthracene with acrylamide, methyl acrylate and acrylic acid has been found to gave ortho-type adducts, but the condensation of allyl alcohol, acrylonitrile and 9-cyanoanthracene gave two isomers. In allyl alcohol gave an oil which could not be induced to crystallize. When this oil was refluxed with acetic anhydride two isomers m.p. 98 oC, 121 oC. The lower melting isomer was identified as ortho-type adducts and the higher melting adduct was therefore assigned the meta-type adducts structur. In the condensation of acrylonitrile and 9-cyanoanthracene gave two isomers, m.p.167 oC, 205 oC The lower melting isomer was identified as ortho-type adducts (9,12-dicyano-9,l0-ethanoanthracene) and the higher melting adduct was therefore assigned the meta-type adducts (9,11-dicyanoethanoanthracen-9,l0-ethanoanthracene).[47]
The condensation of 9-anthraldehyde, 9-methylacetal, 9-aceto-, 9-acetoxy-, 9-cyano-, 9-nitro-, 9-methoxy, 9-chloro- and 9-bromoanthracene with ethylene at temperatures of almost 200 oc can be satisfactory yields (Scheme 5). [48]
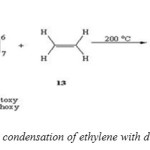 |
(Scheme 5: condensation of ethylene with dienophiles ) Click here to View figure |
The Diels-Alder reaction of 9-substituted anthracene with acetamidoacrylate gave two products the meta adductt has been found and also cycloaddition reaction with ρ-benzoquinone (Scheme 6), N-methyl maleimide.[49]
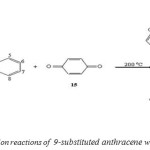 |
(Scheme 6: cycloaddition reactions of 9-substituted anthracene with ρ-benzoquinone) Click here to View figure |
In the Diels-Alder reaction of 9-methylanthracene or 9-chloroanthracene with 2-acetamidoacrylate in nitrobenzene meta isomers were readily obtained in pure form using flash chromatography in moderate to good yields (Scheme 8). The regioselectivity has been determined by ‘H NMR analysis bridgehead proton. The bridgehead proton in the ortho isomer itis a triplet, whereas in the meta isomer is a singlet. Diels-Alder reaction of 2-acetamidoacrylate with 9-chloroanthracene in toluene for 48 h generated adducts in 19 % yield as a 4:1 mixture of meta and ortho isomers. Polar solvents have been shown to increase the reaction rate. for certain Diels–Alder reactions.[50]
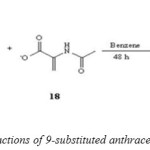 |
(Scheme 7: cycloaddition reactions of 9-substituted anthracene with 2-acetamidoacrylate) Click here to View figure |
Diels–Alder cycloaddition of (R)-9-(N-a-methylbenzylamino) anthracene with N-methylmaleimide and maleic anhydride for 2 h at 80–85 oC, in each case, [51] producing a mixture of two diastereoisomers in excellent conversion and good selectivity (Scheme 8).
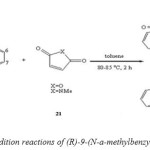 |
(Scheme 8: cycloaddition reactions of (R)-9-(N-a-methylbenzylamino) anthracene) Click here to View figure |
Diels–Alder cycloaddition of 9-methylanthracene with tetranitroethylene refluxing in benzene[52] gave 9-methyl-11,11,12,12-tetranitro-9,l0-dihydro-9,l0-ethanoanthracene. (Scheme 9).
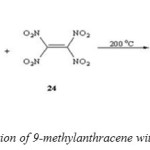 |
(Scheme 9: cycloaddition of 9-methylanthracene with tetranitroethylene ) Click here to View figure |
Diels-Alder reaction of 9-anthracenemethanol with dimethylacetylene-dicarboxylate would lead to the formation of two regioisomeric adducts. (Scheme 10).[53]
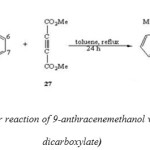 |
(Scheme 10: Diels-Alder reaction of 9-anthracenemethanol with dimethylacetylene-dicarboxylate) Click here to View figure |
Diels-Alder reaction of 9-Nitroantliracene and 9-Anthramide gave both possible adducts with acrylic acid, acrylyl chloride, acrylonitrile and acrylamide with the first two the non-vicinal 9-nitro-11-substituted-9,l0-dihydro-9,l0-ethanoanthracene predominated but with allyl alcohol and methyl acrylate only one adduct was isolated and in each case it was the non-vicinal isomer. The two acrylic acid adducts of 9-nitroanthracene m.p. at 225 oC and 268 oC. Condensed of 9-Anthramide with allyl alcohol at 170-175 oC gave two products of which the major one was the known 12-methylol-9,l0-ethanoanthracene-9 (10 H)-carboxylic acid lactone. The other was identified by heating with acetic anhydride which gave the previously prepared 9-cyano-9,l0-dihydro-9,l0-ethanoanthracene-12-methanol acetate (Scheme 11).[54]
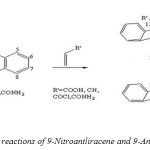 |
(Scheme 11: cycloaddition reactions of 9-Nitroantliracene and 9-Anthramide with dienophiles) Click here to View figure |
The Diels-Alder reaction of 9-substituted anthracene was the key steps for the synthesis of some antidepressant drugs and anxiety such as Benzoctamine, maprotiline[55] and a homologues of these compounds bishomobenzoctamine and bishomomaprotiline.[56-58]
Conclusions
since 100 years of the development of the alder the significant progress in the elucidation of the factors determining the reactivity of the reagents and stability of the reaction products was achieved. The Diels–Alder reactions has become a powerful method of synthesis of the valuable carbo- and heterocyclic products.
These reactions provide a deeper understanding of the special features of other processes in organic synthesis.
References
- Atherton. J, Jones. S, Tetrahedron., 59, 9039 (2003).
- Bouas. L. H, Castellan, A, Desvergne. J and Lapouyade, R, Chem. Soc. Rev., 29, 43 (2000).
- Angelova. A, Van der Auweraer. M, Ionov. R, Vollhardt. D and De Schryver. F, Langmuir., 11, 3167 (1995).
- Landis. C, Parkin. S and Anthony, J. Jpn. J. Appl. Phys., 44, 3921 (2005)
- Suzuki. K, Seno. A, Tanabe. H and Ueno. K, Synthetic Materals., 143, 89 (2004).
- Méry. S, Haristoy. D, Nicoud. J, Guillon. D, Monobe. H, and Shimizu. Y. J, Mater.Chem., 13, 1622 (2003).
- Duff. M, Tan. W, Bhambhani. A, Perrin. B, Thota. J, Rodger. A and Kumar. C, J. Phys. Chem., 110 B 20693 (2006).
- Heiko. I, Eur. J. Org. Chem., 1595 (1999).
- Diels. O and Alder. K., Liebigs Ann. Chem., 460, 98 (1928).
- Diels. O and Alder. K, ibid., 490, 236 (1931).
- Diels. O and Alder. K, Chem. Ber., 62, 554 (1929).
- Mei-Fun. C and Wai-Kee L, Chemical Physics Letters., 368, 630 (2003).
- Kloetzel..M. C Org. React., 1 (1948).
- Diels. 0 and Alder, K. Justus Liebigs Ann. Chem., 450, 237 (1926).
- Sauer. J, Angew. Chem. Int. Ed., 5, 211 (1966).
- Diels. O and Blom. J. H, Koll. W, Ann., 443, 242 (1925).
- Sauer. J, Angew. Chem. Int. Ed., 6, 16 (1967).
- Corey. E, J. Angew. Chem. Int. Ed., 41, 1650 (2002)
- Sauer. J, Angew. Chem., 78, 233 (1966).
- Sauer. J, Wiest. H and Mielert. A, Chem. Ber., 97, 3183 (1964).
- Sustmann. R and Schubert. R, Angew. Chem., 89, 888 (1972).
- Sauer. J and Sustmann. R, Angew. Chem., 92, 773 (1980).
- Houk. K. N and Munchausen. L. L, J. Am. Chem. Soc., 98, 937 (1976).
- Sustmann. R, Bohm. M and Sauer. J, Chem. Ber., 112, 883 (1979).
- Houk. K. N, Li. Y and Ivanchek. J. D, Angew. Chem. Int. Ed. Engl., 31, 682 (1992).
- Rohr. U, Schatz. J and Sauer. J, Eur. J. Org. Chem., 1, 2875 (1998).
- Jenner. G, J. Phys. Org. Chem., 15, 1 (2002).
- Alder. K., Newer Methods of Preparative Organic Chemistry, Vol.1, Wiley-Interscience: New York, 381 (1948).
- Desimoni. G, Tacconi. G, Barco. A and Polloni. G., Natural Product Synthesis through Pericyclic Reactions, ACS Monograph 180, Washington, (1983).
- Carey. F. A and Sundberg. R. J., Advanced Organic Chemistry Part A: Structure and Mechanisms, Second Editiion plenus press, New York, 562 (1984).
- March. J., Advanced Organic Chemistry, Reactions, Mechanisms and Structures. Third Edition, Wiley Eastern, New Delhi, 752 (1987).
- Carruthers, W., Cycloaddition Reactions in Organic Synthesis, Pergamon, Oxford, (1990).
- Fringuelli. F and Tatichi. A., Dienes in the Diels-Alder Reactions, Wiley, New York, (1990).
- Lowry. T. H and Richardson. K. S., Mechanism and Theory in Organic Chemistry; 3rd ed.; Addison-Wesley, New York, 919 (1998).
- Kobayashi. S and Jorgensen. K. A., Cycloaddition Reaction in Organic Synthesis, Wiley, New York, 187, (2001).
- Clayden. J, Greeves. N, Warren. S and Wothers. P., Organic Chemistry, Oxford University press, New York, 914 (2001).
- Fringuelli. F and Tatichi A., The Diels-Alder reaction: Selected practical methods, Wiley, New York, 3, (2002).
- Sauer. J, Angew. Chem., 79, 76 (1967).
- Konovalov. A. I., Usp. Khim., 52, 1852 (1983) [Chem. Rev. USSR., 52 (1983)].
- Kiselev. V. D and Konovalov, A. I, ibid., 58, 383 (1989), [58 (1989) (Eng. Transl.)].
- Konovalov. A. I and Kiselev. V. D., Russ. Chem. Bull. Int. Ed., 52, 293 (2003).
- Kiselev. V. D and Konovalov. A. I, J. Phys. Org. Chem., 22, 466 (2009).
- Peter. V and Alston, J. Org. Chem., 44, 4939 (1979).
- Meek. J. S, Toon. B. T and Cristol. S. J, J. Am. Chem. So., 761 (1952).
- Herndon, W. C. Chem. Rev., 72, 157 (1972).
- Epiotis, N. D. J. Am. Chem. Soc., 95, 5624 (1973).
- Meek. J. S and Dana. J. R, J. Am. Chem. So., 21, 5413 (1956).
- Meek. J. S and Dana. Evans. B, J. Org. Chem., 26, 4281 (1961).
- Singh. M. D and Ningombam. A, Indian Journal of Chemistry. 49B, 789 (2010).
- Yang. B. V and Doweyko. L. M, Tetrahedron Letters., 46, 2857 (2005).
- Ramadan. H, A, Keith A. B, Millan. G. M and Jones. S, Tetrahedron., 18, 1003 (2007).
- Griffin. T. S and Baum. K, J. Org. Chem., 45, 2880 (1980).
- Singh. M. D and Ningombam. A, Indian Journal of Chemistry., 49B, 77 (2010).
- Meek. J. S, Wilgue. D. .R and Dann. J. R, J. Am. Chem. Soc., 82, 2566 (1960).
- Wilhelm. M and Schmidt P, Helv. Chim. Acta., 52, 1385 (1969).
- Karama. U, Al-Saidey. A, Al-Othman. Z and Almansour. A, Molecules. 15, 4201 (2010).
- Al-Saeedi. A, Karama. U and Farooqui. M, Asian Journal of Research in Chemistry., 1460 (2012).
- Al-Saeedi. A, Karama. U and Farooqui. M, Journal of Saudi Chemical Society.,1 (2013).AAsian

This work is licensed under a Creative Commons Attribution 4.0 International License.









