Thin –layer Chromatography of Isomeric Complexes of Ambidentate Ketoanil Ligands: Separation, Detection and Effect of Chelate Ring Contraction on RF Values
Fentahun Adamu1 , Sanidhya Upadhyay2 , Bipin Chandra Upadhyay1 and R. K. Upadhyay1*
1Department of Chemistry, College of Natural and Computational Sciences, Haramaya University, Ethiopia. 2Ranbaxy Research Laboratories, R&DV Department-Analytical Research, Plot No.20, Section-18, Udyog, India
Article Received on :
Article Accepted on :
Article Published : 10 Jun 2013
Complexes of Cu(II) nitrate and chloride salts with p-chloro-, p-bromo-and p-iodo-anils of 2-thiophene glyoxal, isolated as binary mixtures of isomers, were separated on thin-layers of silica gel. Separation of mixtures in bulk and of their known quantity was done by column chromatography for determination of isomeric composition of isolates. Resolved isomers were identified using correlation of their RF with spectral properties. The effect of chelate ring contraction on RF values has also been investigated.
KEYWORDS:Thin layer chromatography; column chromatography; ambidentate ligands; Ketoanils; chelates
Download this article as:| Copy the following to cite this article: Adamu F, Upadhyay S, Upadhyay B. C, Upadhyay R. K. Thin –layer Chromatography of Isomeric Complexes of Ambidentate Ketoanil Ligands: Separation, Detection and Effect of Chelate Ring Contraction on RF Values. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Adamu F, Upadhyay S, Upadhyay B. C, Upadhyay R. K. Thin –layer Chromatography of Isomeric Complexes of Ambidentate Ketoanil Ligands: Separation, Detection and Effect of Chelate Ring Contraction on RF Values. Available from: http://www.orientjchem.org/?p=11977 |
Introduction
Although thin- layer chromatography(TLC), being superior to other techniques in providing rapid and better separation , is used in routine in testing homogeneity, resolution and identification of organic and inorganic compounds as one of the classical methods but mechanistic aspects of analytes migration and diverse interactions of properties of solvents and analytes are usually ignored. Moreover TLC reports on isomeric species generally pertain
to organic compounds whereas inorganic isomers 1-3 including complexes of ketoanils 4,5find little mention in literature. The lack of knowledge on analytical chemistry of isomeric inorganic compounds in general, and of isomeric complexes of ketoanils in particular, tempted us to carry out chromatographic analyses of binary mixtures of isomeric complexes synthesized by Cu( II) chloride and nitrate salts with three ambidentate ligands , 2-thiophene glyoxal-p-chloro-, -p-bromo-and –p-iodo-anils abbreviated as TGCA, TGBA and TGIA respectively, which has not been reported hitherto.
Experimental
Preparation and bulk separation of isomeric complexes
Isolation, stoichemetries and structures of isomeric complexes have been reported in our previous communication 6. Separation of binary isolates in bulk was by column chromatography using silica gel adsorbent and benzene developer. Component ‘a’ of each pair of isomers is slow moving (low RF) and ‘b’ is fast moving (high RF).
Loading and development of TLC plates
Glass plates (20 × 20) coated with silica gel (Merck. KGaA) were used for TLC work. TLC plates were activated by heating at ~ 60 oCto ensure compactness of spots before use. Sample solutions in acetone were spotted on warm plates with fine capillaries in series on a line 2 cm from the lower edge of the plates. The oven dried loaded plates were developed in rectangular glass chambers with ground –in- lids presaturated with developing solvent by ascending technique to obtain reproducible results. When the development had proceeded for 8cm the plates were removed from the chamber and the development time was noted. Owing to dark colours of spots of analytes they were discernible in day light.
Quantitative separation and estimation
Taking advantage of wide difference in RF values (Table-1) of components of binary mixtures in benzene ( low RF, 0.00 and high RF , 0.91-0.95), solutions of binary isolates in acetone containing 200 mg of each were separated by column chromatography conducted in column (50 cm length an 3cm diameter ) containing silica gel ( ~ 65 mesh, BDH) adsorbent. Eluates of fast moving -b ( high RF ) eluted with benzene and slow moving –a (low Rf ) eluted with MeCN were evaporated at ~ 50 oC under reduced pressure and weighed to calculate isomeric composition of binary mixtures ( Table-2). The same experiment was conducted three times.
Results and Discussion
All the non- electrolytic complexes are four coordinate in square planar stereochemistry involving quininoid structure of ligands. Five membered chelate ring of chloro and nitrato Cu( II)-TGCA, Cu( II)-TGBA-a and Cu( II)-TGIA-a complexes is comprised of carbonyl oxygen and azomethine nitrogen atoms whereas chelate ring of CuCl2– TGBA-b, Cu(NO3)2- TGBA-b, CuCl2– TGIA-b and Cu(NO3)2- TGIA-b isomers is composed of carbonyl oxygen and thienyl sulfur 7,8 (Fig.1).
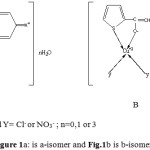 |
Figure 1a: is a-isomer and Fig.1b is b-isomer: Click here to View figure |
Thin layer chromatographic development of all the binary isolates and their resolved individual components was carried out in ten solvent systems and RF values were found the same (Table 1). Although all the solvents, except Me2CO, MeCN and toluene, showed good resolving capacity for binary mixtures of isomeric complexes but best separation could be achieved in benzene.
Para substituents of ligands being electron repellent some electron density is transferred from them to carbonyl oxygen, leading to change from benzenoid to quininoid structure of ligands. The magnitude of this charge on carbonyl oxygen, related directly with electron repelling ability of para substituent of ligands, predominantly govern the metal-oxygen bond strength. The size of the chelate ring, however, is determined by strength of bonds, metal-oxygen and metal-nitrogen bonds, and metal-oxygen and metal-sulphur bonds in low RF and high RF complexes respectively.
In CuCl2-TGCA, Cu (NO3)2–TGCA, CuCl2— TGBA (a), Cu(NO3)2–TGBA (a),CuCl2-TGIA (a) and Cu(NO3)2- TGIA (a) complexes υCu-O and υCu-N frequencies falling in the order of electron repelling ability of para substituent of ligands, Cl (TGCA) > Br (TGBA) > I (TGIA), clearly reveal chelate ring contraction in the order of IR parameters. The Rf values (Table 1) have also been found to follow the same order as to that in chelate ring contraction or spectral parameters in all the solvents except MeCN which showed opposite RF order to spectral parameters probably due to its highest polarity among other solvents used 9.
In high RF (b) complexes of TGBA and TGIA υCu-O and υCu-S frequencies follow the order, Br (TGBA) ≥ I (TGIA), corresponding to electron repelling ability of para substituent, electron density on carbonyl oxygen of ligands or chelate ring contraction. The RF values of these complexes follow the identical order to IR parameters or chelate ring contraction in alcohols but in other solvents, whether oxygen-containing or non-oxygen containing opposite RF order to IR sequence is obtained for the reason unknown as yet.
Both isomers in their mixtures on resolution could be identified by using correlation of metal-ligand bonds frequencies, and RF values in their resolving solvents.
Table 1: Thin layer chromatography of ketoanils and their complexes; spot colour, RF and M-L bond frequencies (cm-1)
| Complexes | Spot colour | RF×100 M-L bond frequencies(cm-1) | |||||||||
| MeOH | EtOH | PrOH | BuOH | Et2O | Me2cO | MeCN | CHCl3 | C6H6 | C6H5CH3 υM-S υM-O υM-N | ||
| TGCA | Light brown | 80 | 92 | 89 | 84 | 62 | 94 | 94 | 34 | 56 | 00 – – – |
| Cu(TGCA)Cl2 | Gray | 92 | 94 | 82 | 44 | 00 | 94 | 93 | 00 | 00 | 00 – 524d 431 |
| Cu(TGCA)(NO3)2 | Gray brown | 88 | 92 | 76 | 77 | 00 | 94 | 92 | 00 | 00 | 00 – 530 425 |
| TGBA | Light brown | 87 | 89 | 82 | 86 | 77 | 94 | 93 | 49 | 57 | 27 – – – |
| Cu(TGBA)Cl2.H2O−a | Gray | 00T | 00 | 00 | 00 | 00 | 90 | 96 | 00 | 00 | 00 – 508 412 |
| Cu(TGBA)Cl2.3H2O−b | Cannary yellow | 64 | 78 | 71 | 74 | 77 | 94 | 96 | 83 | 91 | 00 238 525 – |
| Cu(TGBA)(NO3)2.3H2O−a | Gray | 00T | 00 | 00 | 00 | 00 | 90 | 96 | 00 | 00 | 00 – 525 425 |
| Cu(TGBA)(NO3)2−b | Cannary yellow | 62 | 74 | 76 | 64 | 76 | 90 | 96 | 85 | 91 | 00 249 482 – |
| TGIA | Light brown | 84 | 93 | 89 | 80 | 76 | 94 | 95 | 48 | 51 | 28 – – – |
| Cu(TGIA)Cl2.3H2O−a | Gray brown | 00T | 00 | 00 | 00 | 00 | 94 | 98 | 00 | 00 | 00 – 496 411 |
| Cu(TGIA)Cl2−b | Cannary yellow | 57 | 76 | 64 | 67 | 81 | 94 | 98 | 97 | 95 | 00 240d 525 – |
| Cu(TGIA)(NO3)2−a | Gray brown | 00T | 00T | 00 | 00 | 00 | 97 | 00 | 00 | 00 | 00 – 525 405 |
| Cu(TGIA)(NO3)2−b | Cannary yellow | 59 | 72 | 63 | 67 | 85 | 94 | 97 | 95 | 95 | 00 250 475 – |
Development time (min) 25 27 45 60 30 9 19 20 22 24
Table -2 Isomeric composition of binary mixtures
|
Mixture of components resolved
|
Amount of mixture applied(mg) | Amount of mixture components recovered(mg) |
% Eror |
Isomeric composition |
| Cu(TGBA)Cl2.H2O (a ) | 138 | |||
| Cu(TGBA)Cl2.3H2O (b) | 200 | 57 | 2.5% | 121:50 |
| Cu(TGBA)(NO3)2.3H2O (a) | 156 | |||
| Cu(TGBA)(NO3)2 (b) | 200 | 42 | 1:1% | 371:100 |
| Cu(TGIA)Cl2.3H2O (a) | 100 | |||
| Cu(TGIA)Cl2 (b) | 200 | 100 | 0:0% | 1:1 |
| Cu(TGIA)(NO3)2 (a) | 140 | |||
| Cu(TGIA)(NO3) 2(b) | 200 | 58 | 1.0% | 241:100 |
The amounts of mixture components recovered are the mean values of three experiments
References
- Kaufmann, G.B., J.Chromatogr. 117: 455 (1976)
- Kaufmann, G.B., Benson, B.W., J. Inorg. Chem. 6:411 (1967)
- Tsunoda, T., Takeuchi, T., Yoshino, Y. Sci.paperscall.Gen.Educ.,Univ.of Tokyo 14: 55 (1964)
- Upadhyay, R.K., Kumari, V., Singh, V. P., J.liquid Chrmatogra. 5: 1141(1982)
- Upadhyay, R.K., Bajpai, A.K., Rathore, K., Chromatographia, 18: 618(1984)
- Adamu, F., Upadhyay, R.K., Ayalew, A., Bulgarian Chem.Comm. Communicated (2011)
- Upadhyay, R.K., Rani, A., Acta Chim.Hung., 124: 629 (1987)
- Upadhyay, R.K., D.Sc.Thesis, C.C.S. Univ., Meerut (1994)
- Upadhyay, R.K., Chromatographia, 25: 324(1988)

This work is licensed under a Creative Commons Attribution 4.0 International License.









