Thin-Layer Chromatography of Imino-oxazolidinones: Separation, Identification and Estimation
Tekle weyni Assefa ssefa1, Tofi k Ahmed1, Sanidhya anidhya Upadhyay adhyayadhyay2 and Raj Kumar Upadhyay adhyayadhyay1*
1Department of Chemistry, Collage of Natural and Computational Science, Haramaya University, Ethiopia. 2Ranbaxy Research Laboratories, R and DV, Department Analytical Research, Plot No-20, Section 18, Udygo, India. Corresponding Author E-mail: rkupadhyay14@gmail.com
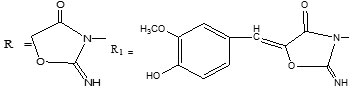
Imino-oxazolidinones, the cyclocondensation products of chloroacetanilides with potassium cyanate and their condensation products with vanillin including isomers have been chromatographed on starch bound silica gel thin-layers using one and two component solvent systems and effects of various properties of developing solvents and migrating species on RF values have been investigated in addition to the separation, identification and estimation.
KEYWORDS:isomers; iminooxazolidinones; Thin layer chromatography
Download this article as:| Copy the following to cite this article: ssefa T. W. A, Ahmed T. K, adhyayadhyay S. A. U, adhyayadhyay R. K. U. Thin-Layer Chromatography of Imino-oxazolidinones: Separation, Identification and Estimation. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: ssefa T. W. A, Ahmed T. K, adhyayadhyay S. A. U, adhyayadhyay R. K. U. Thin-Layer Chromatography of Imino-oxazolidinones: Separation, Identification and Estimation. Orient J Chem 2013;29(2). Available from: http://www.orientjchem.org/?p=22052 |
Introduction
Although thin-layer chromatographic (TLC) studies on heterocyclic organic compounds including thiazolidinones are well documented and numerous reports are also available on isomers but imino-oxazolidinones have not been investigated hitherto. This aroused our interest to carry out TLC studies, as this technique is superior to others in providing rapid and better separation, on a few imino-oxazolidinones including isomers. The effect of various properties of developing solvents and migrating species on RF values and separation of isomers were investigated. The relationship between λ max and infrared spectral frequencies of characteristic group(s) and RF values were used in the identification of compounds in their mixture(s) in resolving solvents.
Experimental
Materials
Imino-oxazolidinones, obtained by cyclocondensation of chloroacetinilides with potassium cyanate in dry ethanol followed by refluxing for ~ 2 h, washing of residues with water, and their condensation products obtained by their reaction with vanillin in dry ethanol in the presence of anhydrous sodium acetate followed by refluxing for 3-4 h, were purified by double crystallization from acetone. The composition of each product was confirmed by elemental analysis, molecular weight determination (Rast method), and infrared, 1H and 13C NMR spectroscopy as reported elsewhere.
In synthetic work reagents grade chemicals (95-97%) were used as supplied where as in TLC studies analytical (AR)/ HPLC-grade solvents were used.
Procedures
Glass plates (2020 cm) were coated by spreading an aqueous slurry containing homogeneous mixture of silica gel (BDH) and starch (19:1, w/w) with a laboratory- built applicator and coated plates were air dried. Both sides of gel layer were scraped of to a width of 5 mm and the coated plates were activated by heating ca.800C for 1h before use.
In order to ensure the compactness of spots spotting of standard solution of samples in acetone was done on warm plates. For qualitative studies sample solutions were spotted as small drops using fine glass capillaries on 0.1 cm thick layer whereas in quantitative work known volumes of solutions were applied with a micropipette to a 0.2 cm thick gel layers. Sample solutions were applied as a series of spots or bands in a line 2 cm from the edge of the plate. Oven dried loaded plates were developed in rectangular glass chamber, pre-saturated with developing solvent to ensure reproducibility of results, with ground- in- lids by ascending technique. On development for ca. 8 cm the plates were removed from the chamber. Although all the spots were visualized in day light, some were more clearly discernible in iodine vapor.
For quantitative analysis chromatogram bands were scraped off, centrifuged with 1-2ml acetone three times and volume was made up to 5 ml. The absorbance of the solution of each mixture component was measured on SP-65 Uv-Vis spectrophotometer at the wave length of maximum absorption (λ max) of the solute and concentration was calculated from linear calibration curves obtained in the range of 0-400 µg under identical conditions of medium ( acetone ) and temperature (2020C).
Results and Discussion
Effects of various parameters on RF values
The effect of gel layer thickness and presence of other compounds (mixture) on RF values were examined for some isomeric oxazolidinones as migrating spots in resolving solvents. The RF data for ternary and binary mixtures of isomers in Table 2 and 3 show a lowering of RF values with increase in gel layer thickness whereas almost identical RF values obtained when the compounds migrating individually (table 1) and in mixture (table2) results that the migration is independent of the presence of other compounds.
The effect of solvents polarity on RF values was studied with all oxazolidinones in oxygen- containing solvents. The order of RF values, BuOH PrOH EtOH MeOH corresponding to the solvent polarity, shows that RF values and their sequence is governed by the solvent polarity. In non-oxygen-containing solvents order of RF values CHCl3 C6H6 petroleum ether n-hexane, being consistent with the sequence in their dielectric constants, supports the inference arrived on oxygen-containing solvents. The higher RF values in aromatic solvent benzene than in aliphatic solvent containing same carbon atoms such as n- hexane clearly show that aromatic solvents are better than the aliphatic solvents in establishing RF values because double bonds of benzene forms weak hydrogen bonds.
TLC studies of isomeric ternary nitro- and methoxy- oxazolidinone mixtures revealed particular RF orders depending on the nature of the solvent, i.e. whether it is oxygen-containing or non- oxygen- containing or it is a one or two component system. In almost all the oxygen-containing solvents irrespective of the nature of functional groups and polarity of solvents, compounds of both the series exhibit RF order p <; in mixture solvents the same order is established generally. This reveals that position of substituted group or steric hindrance in solutes mainly governs the RF value irrespective to other properties of solutes and solvents.
To study the effect of nature of substituent on RF values, p-substituted oxazolidinones were chosen as in this position the steric effects are smaller than in the other positions and in such cases the nature of substituent group predominates. From the higher RF values of nitro substituted oxazolidinones than the methoxy compounds, the effect of electron withdrawing nature of the substituted groups (NO2 OCH3) is evident.
The effect of functional groups of solvent on RF values has been studied in MeOH and MeCOOH. The higher RF values of all the compounds in MeOH than in MeCOOH clearly reveal better effect of alcoholic group on RF values than carboxylic group. In two component mixture solvents of n–hexane–EtOAc (2:1,1:1 and 1:2,v/v) ratio RF values of all the migrating compounds are intermediate to the RF values obtained in pure solvents as expected but benzene –EtOH mixture solvents (3:2,1:1and 2:3,v/v) showed abnormal RF values (higher than pure solvents). The exceptionally higher values in benzene –EtOH (3:2, 1:1 and 2:3, v/v) mixtures could be attributed to the formation of hemiaketals or ketals by deprotonated ethanol in the presence of benzene inert medium with ketonic group(s) of the oxazolidinones. However in benzene–EtOH (4:1, v/v) the RF values lying in between pure solvents values could most probably be due to inadequate quantity of EtOH as compared to benzene to form the hemiketals or ketals.
Separation, identification and determination of oxazolidinones in mixture
Among different solvents tried for separation of oxazolidinones, chloroform showed the highest resolving capacity as it could resolve several mixtures of six compounds; the best resolution of diverse mixtures of three compounds, however, could only be achieved in benzene, n-hexane–EtoAc (1:1,v/v) and benzene–EtOH (4:1,v/v). The ternary and binary mixtures of isomeric compounds of both series (R and R1) have also been resolved only by these solvents (Table 2).
The IR stretching frequency of the C=NH, a common characteristic group, and λ max of the isomeric oxazolidinones have been correlated with the RF values in their resolving solvents. In R-C6H4-NO2 isomers RF order, meta para ortho is similar to that of the λ max and C=NH stretching values whereas in R-C6H4-OCH3 isomers the RF values fall in opposite order to that of their λ max values. In binary mixtures of R1-C6H4-NO2 and R1-C6H4-OCH3 isomers the RF and λ max values as well as the C=NH frequencies are in identical orders. These RF and spectral correlations were used for the identification of mixture components after separation.
In order to test the application of the TLC method in the analysis of oxazolidinones various mixtures of isomeric compounds were analysed qualitatively (table 2) on 0.1cm and quantitatively on 0.2cm thick layer (Table 3). The maximum amounts of isomers resolved from their mixture reveal the maximum separation limit of this method. All the results are reproducible.
Table 1: Color, Rf , λ max and IR frequencies values of analysed compounds in various one and two solvent systems.
| Compound
|
Spot color | λ max values | IR frequencies
(cm -1) |
RF100 |
||||||||||
| ۷HC=N | MeOH | EtOH
|
PrOH | BuOH | Et2O | EtOAc | AcOH | Petroleum ether | n-Hexane | CHCl3 | Benzene | |||
| R-C6H4-NO2(o) | Yellow green | 360 | 1595 | 89 | 84 | 83 | 86 | 60 | 91 | 87 | 00 | 00 | 73 | 40 |
| R-C6H4-NO2(m) | olive | 350 | 1591 | 88 | 83 | 80 | 72 | 61 | 90 | 86 | 00 | 00 | 35 | 39 |
| R-C6H4-NO2(p) | Yellow green | 360 | 1595 | 88 | 82 | 79 | 85 | 65 | 89 | 85 | 00 | 00 | 45 | 31 |
| R-C6H4-OCH3(o) | Alice blue | 340 | 1684 | 88 | 83 | 84 | 76 | 63 | 86 | 75 | 00 | 00 | 46 | 10 |
| R-C6H4-OCH3(m) | Yellow green | 350 | 1665 | 87 | 82 | 82 | 69 | 63 | 84 | 73 | 00 | 00 | 22 | 00 |
| R-C6H4-OCH3(p) | Light yellow | 345 | 1645 | 86 | 81 | 80 | 66 | 56 | 83 | 68 | 00 | 00 | 30 | 19 |
| R1-C6H4-NO2(o) | yellow | 355 | 1598 | 87 | 82 | 83 | 74 | 70 | 90 | 76 | 00 | 00 | 33 | 21 |
| R1-C6H4-NO2(m) | Orange red | 300 | 1593 | 84 | 81 | 81 | 77 | 64 | 88 | 74 | 00 | 00 | 24 | 24 |
| R1-C6H4-NO2(p) | yellowish | 430 | 1632 | 80 | 79 | 74 | 89 | 62 | 83 | 73 | 00 | 00 | 36 | 22 |
| R1-C6H4-OCH3(o) | yellow | 360 | 1664 | 90 | 82 | 81 | 67 | 65 | 85 | 75 | 00 | 00 | 28 | 10 |
| R1-C6H4-CH3(m) | Green yellow | 330 | 1590 | 87 | 81 | 76 | 71 | 60 | 84 | 70 | 00 | 00 | 28 | 08 |
| R1-C6H4-OCH3(p) | brown | 350 | 1595 | 86 | 80 | 74 | 80 | 58 | 82 | 68 | 00 | 00 | 32 | 00 |
| Development time | 25 | 42 | 45 | 30 | 15 | 12 | 10 | 10 | 10 | 10 | 15 | |||
Continued..
|
RF 100 |
||||||
| n-hex–Ethyl acetate(v:v)
|
Benzene- Ethanol(v:v) | |||||
|
2:1 |
1:1 |
1:2 |
4:1 | 3:2 |
1:1 |
2:3 |
|
34 |
66 |
87 |
82 |
86 |
95 |
92 |
|
31 |
62 |
85 |
81 |
85 |
85 |
90 |
|
23 |
61 |
86 |
78 |
86 |
94 |
89 |
|
21 |
59 |
79 |
63 |
87 |
92 |
89 |
|
30 |
62 |
80 |
57 |
88 |
93 |
90 |
|
28 |
50 |
77 |
55 |
88 |
91 |
89 |
|
32 |
60 |
85 |
84 |
86 |
96 |
92 |
|
33 |
53 |
78 |
78 |
85 |
92 |
94 |
|
29 |
59 |
80 |
78 |
86 |
90 |
95 |
|
30 |
45 |
77 |
79 |
87 |
96 |
88 |
|
29 |
77 |
70 |
41 |
88 |
90 |
87 |
|
23 |
48 |
71 |
38 |
87 |
79 |
87 |
|
25 |
20 |
17 |
36 |
27 |
26 |
32 |
Table 2: Qualitative resolution of isomeric mixtures.
| No | Mixture of isomeric oxazolidinones | Resolving solvent |
| 1 | R-C6H4-NO2(o) or R-C6H4-NO2(p) + R-C6H4-NO2(m)
(86) (85) (72) |
Bu OH |
| 2 | R-C6H4-OCH3(m) + R-C6H4-OCH3(p)
(62) (50) |
n-hexane-EtOAs (1:1,v/v) |
| 3 | R1-C6H4-NO2(o) or R1-C6H4-NO2(m) + R1-C6H4-NO2(p)
(74) (77) (89) |
Bu OH |
| 4 | R1-C6H4-OCH3(o) + R1-C6H4-OCH3(m) or R1-C6H4-OCH3(p)
(79) (41) (38) |
Benzene –EtOH (4:1,v/v) |
| 5 | R-C6H4-NO2(o) + R-C6H4-NO2(m) + R-C6H4-NO2(p)
(73) (35) (45) |
CHCl3 |
| 6 | R-C6H4-OCH3(o) + R-C6H4-OCH3(m) + R-C6H4-OCH3(p)
(46) (22) (30) |
CHCl3 |
Values in parenthesis are Rfx100
Table 3: Quantitative analysis of isomeric oxazolidinones
Experiment.1a
| Isomeric oxazolidinones in mixture | Amount loaded (µg) | Amount recovered(µg) | Mean | SD* | RSD** | Error (%) | RF x 100
|
Resolving solvent |
| R-C6H4-NO2(o) | 200 | 198.8, 199.4, 200 | 199.4 | 0.63 | 0.31 | 0.31 | 70 |
CHCl3 |
| R-C6H4-NO2(m) | 250 | 248.3, 249.6, 249.2 | 249.1 | 0.64 | 0.26 | 0.39 | 32 | |
| R-C6H4-NO2(p) | 250 | 247.7, 245, 244.95 | 245. 9 | 1.5 | 0.65 | 1.64 | 42 | |
| R-C6H4-OCH3(o) | 200 | 200, 196.9, 197.5 | 198.1 | 1.65 | 0.83 | 0.94 | 43 |
CHCl3 |
| R-C6H4-OCH3(m) | 250 | 250, 238.1, 244.4 | 244.2 | 5.94 | 2.43 | 2.3 | 20 | |
| R-C6H4-OCH3(p) | 200 | 199.6, 198.3, 194.2 | 197.4 | 2.84 | 1.44 | 1.3 | 28 | |
| R1-C6H4-NO2(o) | 200 | 39.92, 39.5, 38.58 | 196.7 | 3.47 | 1.8 | 1.7 | 71 | BuOH |
| R1-C6H4-NO2(p) | 250 | 249.5, 243.6, 248.2 | 247.1 | 3.1 | 1.3 | 1.2 | 85 | |
| R1-C6H4-OCH3(o) | 200 | 198.9, 199.5, 196.2 | 198.2 | 1.78 | 0.90 | 0.93 | 78 | Benzene –EtOH(4:1,v/v) |
| R1-C6H4-OCH3(m) | 250 | 249.4, 243.1,244.2 | 245.6 | 3.3 | 1.4 | 1.8 | 39 | |
| R1-C6H4-OCH3(o) | 200 | 198.9, 199.5, 196.2 | 198.2 | 1.78 | 0.90 | 0.93 | 78 | Benzene –EtOH(4:1,v/v) |
| R1-C6H4-OCH3(p) | 250 | 249.4, 243.1, 244.2 | 247.6 | 1.9 | 0.79 | 0.96 | 35 |
SD*standard deviation RSD** relative standard deviation
Experiment .2a
| Isomeric oxazolidinones in mixture | Amount loaded
(µg) |
Amount recovered(µg) | Mean | Mean | SD* | RSD** | Error (%) | Resolving solvent |
| R-C6H4-NO2(o) | 150 | 148.1, 148.7, 150 | 148.9 | 0.95 | 0.64 | 0.69 | 70 |
CHCl3 |
| R-C6H4-NO2(m) | 250 | 250,248.75,249.27 | 249.3 | 0.64 | 0.26 | 0.28 | 32 | |
| R-C6H4-NO2(p) | 200 | 195, 199.54, 195 | 196.5 | 2.62 | 1.33 | 1.7 | 42 | |
| R-C6H4-OCH3(o) | 150 | 150, 147.5, 148.13 | 148.5 | 1.30 | 0.88 | 0.94 | 43 |
CHCl3 |
| R-C6H4-OCH3(m) | 250 | 248.8, 244.4, 244.375 | 245.8 | 2.53 | 1.03 | 1.7 | 20 | |
| R-C6H4-OCH3(p) | 150 | 150, 148.3, 147.97 | 148.8 | 1.10 | 0.74 | 0.8 | 28 | |
| R1-C6H4-NO2(o) | 150 | 150, 148.8, 147.1 | 148.6 | 1.46 | 0.99 | 0.93 | 71 | BuOH
|
| R1-C6H4-NO2(p) | 200 | 198.2, 192.7, 193.2 | 194.7 | 3 | 1.6 | 2.7 | 85 | |
| R1-C6H4-OCH3(o) | 150 | 148.9, 148.9, 143.3 | 147 | 3.2 | 2.2 | 2 | 78 | Benzene-EtOH(4:1, v/v) |
| R1-C6H4-OCH3(m) | 250 | 246.9, 245.6, 245 | 245.8 | 0.95 | 0.39 | 1.7 | 39 | |
| R1-C6H4-OCH3(o) | 150 | 148.9, 148.9, 143.3 | 147 | 3.2 | 2.2 | 2 | 78 | Benzene-EtOH(4:1, v/v) |
| R1-C6H4-OCH3(p) | 250 | 246.9, 245, 245.6 | 248. 9 | 3.1 | 3.1 | 0.44 | 35 |
SD*standard deviation RSD** relative standard deviation
Experiment .3a
| Isomeric oxazolidinones | Amount loaded
(µg) |
Amount recovered(µg) | Mean | SD* | RSD** | Error (%) | RFx100 |
Resolving solvent |
| R-C6H4-NO2(o) | 100 | 98.125, 99.4, 98.75 | 98.75 | 0.625 | 0.63 | 1.25 | 70 | CHCl3 |
| R-C6H4-NO2(m) | 250 | 248.8, 249.5, 247.9 | 248.8 | 0.83 | 0.34 | 0.5 | 32 | |
| R-C6H4-NO2(p) | 150 | 149.5, 148.6, 148.2 | 148.8 | 0.69 | 0.47 | 0.81 | 42 | |
| R-C6H4-OCH3(o) | 100 | 100, 98.75, 98.125 | 98.96 | 0.95 | 0.96 | 1.04 | 43 | CHCl3 |
| R-C6H4-OCH3(m) | 250 | 249.4, 238.1, 248.1 | 245.2 | 6.2 | 2.5 | 1.9 | 20 | |
| R-C6H4-OCH3(p) | 100 | 99.2, 98.3, 94.2 | 97.2 | 2.7 | 2.8 | 2.8 | 28 | |
| R1-C6H4-NO2(o) | 100 | 99.6, 96.7, 97.5 | 97.9 | 1.50 | 1.5 | 2.1 | 71 | BuOH |
| R1-C6H4-NO2(p) | 150 | 148.2, 148.2, 144.1 | 146.8 | 2.4 | 1.6 | 2.1 | 85 | |
| R1-C6H4-OCH3(o) | 100 | 98.9, 99.4, 98.3 | 98.9 | 0.56 | 0.56 | 1.1 | 78 | Benzene-EtOH(4:1, v/v) |
| R1-C6H4-OCH3(m) | 200 | 200, 193.8, 198.8 | 197.5 | 3.30 | 1.7 | 1.3 | 39 | |
| R1-C6H4-OCH3(o) | 100 | 98.9, 99.4, 98.3 | 98.9 | 0.56 | 0.56 | 1.1 | 78 | Benzene-EtOH(4:1, v/v) |
| R1-C6H4-OCH3(p) | 200 | 200, 193.8, 198.8 | 197.96 | 2.57 | 2.57 | 1 | 35 |
SD*standard deviation RSD** relative standard deviation
Acknowledgment
The author wishes to acknowledge Haramaya University for financial support.
References
- R. K. Upadhyay, N. Agarwal and N. Gupta, J.Chromatogr. 542 (1991) 531.
- R.K. Upadhyay, R.K. Sharma, G.Babu and G. Misbra , J. planar chromatogr. 7 (1994) 464.
- T. Bufabo, A.Tadesse, R.K. Upadhyay, J. pharm- Res (2012) communicated.
- E. Stahl, Thin- layer chromatography, Springer, Berlin, 2nd ed., 1966, p.56.
- X. Ziemeng and L.Yuan, J. Spectrochimica, Acta 58 (2002

This work is licensed under a Creative Commons Attribution 4.0 International License.









