Synthesis of 1, 4-Dihydropyridine Derivatives using FeCl3 as Catalyst under Solvent-free Condition
Abdorrahman Keyhani and Farhad Hatamjafari
Department of Chemistry, Faculty of Science, Islamic Azad University-Tonekabon Branch, Tonekabon, Iran.
Corresponding Author E-mail: keyhaniabdorrahman@yahoo.com
Article Received on :
Article Accepted on :
Article Published : 10 Jun 2013
A mixture of ethyl acetoacetate, benzaldehyde and ammonium acetate and in the presence of FeCl3 under solvent-free condition were converted to 1, 4-dihydropyridines with good yields.
KEYWORDS:1; 4-dihydropyridines; ammonium acetate; ethyl acetoacetate; FeCl3
Download this article as:| Copy the following to cite this article: Keyhani A, Hatamjafari F. Synthesis of 1, 4-Dihydropyridine Derivatives using FeCl3 as Catalyst under Solvent-free Condition. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Keyhani A, Hatamjafari F. Synthesis of 1, 4-Dihydropyridine Derivatives using FeCl3 as Catalyst under Solvent-free Condition. Available from: http://www.orientjchem.org/?p=11980 |
Introduction
Chemistry of dihydropyridines in 1882 with Arthur Hansh reports the β-keto ester, aldehydes and ammonia can be combined to form 1, 4-dihydropyridine was developed. Because of the many benefits of 1, 4-dihydropridine is presented and now as vital medicines to treat angina, high blood pressure and allergies have been introduced. The combination of amlodipine and infedipines as brand name drugs1-5. Another successful treatment of 4-aryl -1, 4-dihydropridine is that they use as calcium channels blockers in the treatment of cardiovascular disease and hypertension. Calcium channel blockers are a class of drugs that control entrance of calcium into cells and exit of calcium from cellular stores. These compounds lower blood pressure by relaxing smooth muscle wall of heart and arteries and reduce their external resistance. According to the various applications of these compounds , different method for synthesis of derivatives of 1, 4-dihydroprydines in different conditions and different catalysts are presented ,considering that some of these methods are time consuming, less economical and are harmful for the environment, provide appropriate methods, with short time, high efficiency, environmental friendly is valuable5-11.
Previously, we have synthesized a number of heterocyclic compounds12-20. Although numerous methods are capable of affecting these synthesis has been previously reported. FeCl3 has been used previously as a catalyst for synthesis of organic compound21, herein we report FeCl3 a new catalyst for the synthesis of 1, 4-DHPs at one pot reaction with high yields and easy separation under solvent-free condition (Scheme 1).
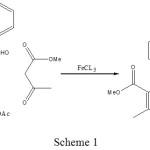 |
Scheme 1 |
Experimental
General Procedure for the Preparation of 1, 4-dihydropyridine derivatives
A mixture of ethyl acetoacetate (2 mol), aromaticaldehyde (1 mol) and ammonium acetate (1 mol) and FeCl3 (0.004 g) was refluxed in 110oC for 1h. The obtained solid was filtered; the solid was washed with water and recrystallized using absolute ethanol (5a-f).
Compound 5a: Diethyl 1,4-Dihydro-2,6-Dimethyl-4-Phenylpyridine-3,5-Dicarboxylate
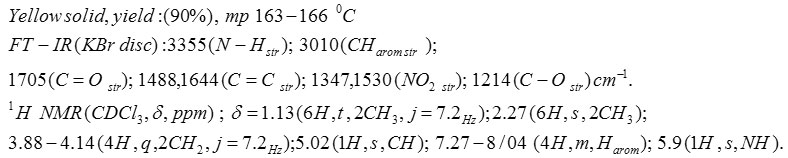
Compound 5b: Diethyl 1,4-Dihydro-2,6-Dimethyl-4-(3-Nitrophenyl)Pyridine-3,5-Dicarboxylate
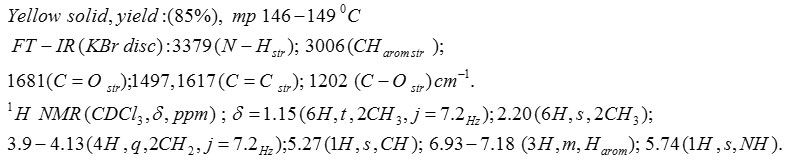
Compound 5C: Diethyl 4-(2,4-Dichlorophenyl)-1,4-Dihydro-2,6-Dimethylpyridine-3,5-Dicarboxylate
Compound 5d: Diethyl 1,4-Dihydro-2,6-Dimethyl-4-M-Tolylpyridine-3,5-Dicarboxylate
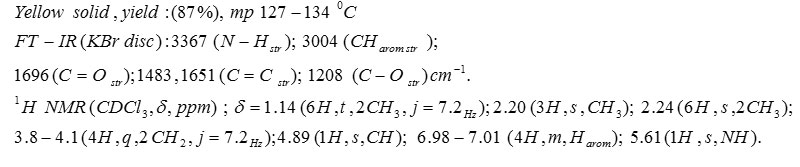 Compound 5e: Diethyl 4-(4-(Dimethylamino)Phenyl)-1,4-Dihydro-2,6-Dimethylpyridine-3,5-Dicarboxylate
Compound 5e: Diethyl 4-(4-(Dimethylamino)Phenyl)-1,4-Dihydro-2,6-Dimethylpyridine-3,5-Dicarboxylate
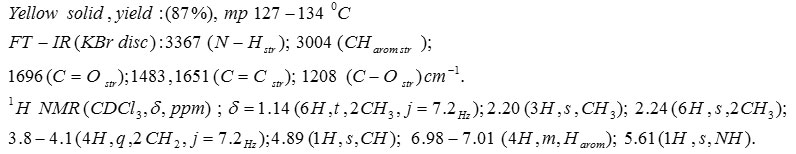
Compound 5f: Diethyl 1,4-Dihydro-2,6-Dimethyl-4-(4-Nitrophenyl)Pyridine-3,5-Dicarboxylate

Results and Discussion
Herein, we report FeCl3 as catalyst which could provide an efficient, cheap, easy separation, high yield and simple route under solvent-free condition for the synthesis of 1,4-DHPs.
Acknowledgements
We gratefully acknowledge the financial support from the Research Council of Tonekabon Branch Islamic Azad University.
References
- David J. T., Biochemical Pharmacology., 74: 1-9 (2007).
- Love B., Goodman M., Snader K., Tedeschi R and Macko E., J. Med. Chem., 17: 956 (1974).
- Bossert F., Meyer H and Wehinger E., Angew. Chem. Int. Ed. Engl., 20: 762 (1981).
- Breitenbucher J.G and Figliozz G., Tetrahedron Lett., 41: 4311 (2000).
- Boer R and Gekeler V., Drugs Fut., 20: 499 (1995).
- Wang L.M., Sheng J. Zhang L., Han J.W., Fan Z.Y., Tian H and Qian C.Y., Tetrahedron., 61: 1539 (2005).
- Ji S.J., Jiang Z.Q., Lu J and Loh T.P., Synlett., 831 (2004).
- Ko S., Sastry M.N.V., Lin, C and Yao, C.F., Tetrahedron Lett., 46: 5771 (2005).
- Dzvinchuk I.B and Tolmacheva, N.A., Chem. Heterocycl. Comp., 37: 506 (2001).
- Chebanov V.A., Saraev V.E., Kobzar K.M., Desenko S.M., Orlov, V.D and Gura, E.A., Chem. Heterocycl. Comp., 40: 475 (2004).
- Bidram A., Hatamjafari F and Doryeh A., Orient. J. Chem., 29: (2013 in press)
- Azizian J., Hatamjafari F., Karimi A. R. and Shaabanzadeh M., Synthesis. 5: 765 (2006).
- Azizian J., Shaabanzadeh M., Hatamjafari F. and Mohammadizadeh M.R., Arkivoc., (xi): 47 (2006).
- Hatamjafari F., Synthetic Communications., 36: 3563 (2006).
- Azizian J., Hatamjafari F. and Karimi A. R., Journal of Heterocyclic Chemistry., 43: 1349 (2006).
- Hatamjafari F and Montazeri N., Turkish Journal of Chemistry., 33: 797 (2009).
- Hatamjafari F., Orient. J. Chem., 28: 141 (2012).
- Hatamjafari F., Orient. J. Chem., 29: (2013 in press).
- Hatamjafari F and Alijanichakoli F., Orient. J. Chem., 29: (2013 in press).
- Hatamjafari F and Hosseinian A., Orient. J. Chem., 29: (2013 in press).
- Lu J and Bai Y., Synthesis., 466 (2002).

This work is licensed under a Creative Commons Attribution 4.0 International License.










