Synthesis, Characterization and Biological Evaluation of Transition and Inner Transition Metal Complexes of Ligands Derived Schiff Base from 1-phenyl-2, 3-dimethyl-4-(4-iminopentan-2-one)-pyrazole-5-one and 2-aminophenol
Anubha Saxena and Rajneesh Saxena
Department of Chemistry, S. M. College, Station Road, Chandausi - 202 412, India.
DOI : http://dx.doi.org/10.13005/ojc/290228
The ligand derived from 1-phenyl-2, 3-dimethyl-4-(4-iminopentan-2-one)-pyrazolin-5-one and 2-aminophenol behaved in neutral tetra dentate manner. Its complexes with Ti (III), V (III), Mn (III), Ru (III), MoO (V), MoO2 (VI), and UO2 (VI) were prepared and characterized by elemental analysis, conductivity, magnetic studies and spectral data. Based on these studies octahedral geometry has been proposed for all these complexes. The ligand and selected transition metal complexes have also been evaluated for antimicrobial activity against S. aureus and B. subtilis. From the biological data, we can infer that Schiff base of 4-aminoantipyrin has better antimicrobial activity in comparison to the native one. Also, the antimicrobial activity is highly influenced by the nature of the metal ion. The order of antibacterial activities against S. aureus and B. subtilis are MoO (V) > MoO2 (VI) > UO2 (VI) H” Ru (III) > Mn (III) > V (III) > Ti (III) and Mn (III) > MoO (V) > MoO2 (VI) > Ru (III) > UO2 (VI) > V (III) > Ti (III) respectively.
KEYWORDS:Schiff base; transition metal complex; ligand; antimicrobial and octahedral
Download this article as:| Copy the following to cite this article: Saxena A, Saxena R. Synthesis, Characterization and Biological Evaluation of Transition and Inner Transition Metal Complexes of Ligands Derived Schiff Base from 1-phenyl-2, 3-dimethyl-4-(4-iminopentan-2-one)-pyrazole-5-one and 2-aminophenol. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Saxena A, Saxena R. Synthesis, Characterization and Biological Evaluation of Transition and Inner Transition Metal Complexes of Ligands Derived Schiff Base from 1-phenyl-2, 3-dimethyl-4-(4-iminopentan-2-one)-pyrazole-5-one and 2-aminophenol. Orient J Chem 2013;29(2). Available from: http://www.orientjchem.org/?p=22171 |
Introduction
The term Schiff base was invented by Hugo Schiff for the condensation reaction between an aldehyde and an amine. Schiff base ligands are able to coordinate metals through imine nitrogen and another group usually linked to aldehyde or ketone [1, 2]. Schiff base complexes incorporating phenolic group as chelating moieties in the ligand are considered as models for executing important biological reactions and mimic the catalytic activities of metalloenzymes [3, 4]. Recently, the Schiff bases ligand containing various donor atoms (like N, O, S etc.) and their metal complexes have been shown broad range of their application as biological, biochemical, analytical, antimicrobial, antibacterial, anticancer, antitumor and antifungal activity [5-9]. They used as catalyst, in medicine like antibiotics and anti-inflammatory agents and in the industry as anticorrosion. The synthesis of transition metal complexes with Schiff base ligands are considered due to sensitivity, selectivity and synthetic flexibility towards metal atoms [10]. It is known that the existence of metal ions bonded to biologically active compounds may increase their activities [11, 12]. In recent years, because of new interesting applications found in the field of medicine, the metal complexes with tridentate O, N, N types of alternative structures have attracted the attention of chemist. In this paper we describe the synthesis, characterization and behavior of the tetradentate Schiff bases of 4-aminoantipyrin ligands with various metal complexes. The structures of all these complexes have been investigated by using elemental analysis, FTIR, elemental analysis, magnetic susceptibility and conductivity measurements. Although 4-aminoantipyrin itself exhibits antimicrobial activity, its activity can be enhanced by formation of its Schiff’s bases by condensation with aldehydes, ketones, carbazides etc. Antibacterial activities of these complexes were determined as MICs values using the micro dilution broth method against gram-positive bacteria, Staphylococcus aureus and Bacillus subtilis.
Material and Methods
Physical Measurement
All the chemicals and solvents used were of highest purity or A.R. grade. The metal salts used were TiCl3, VCl3 MnCl3, RuCl3, MoOCl3, MoO2Cl2 and UO2 (NO3)2. TiCl3 was prepared in the lab by literature method while all other metal salts were procured from Merck and used as such. The solvents were carefully purified before use. Melting points of the ligand and its metal complexes were determined in an Electro thermal 9200 by open capillary method. The IR spectra (Nujol/KBr) were recorded in the range 400- 4000 cm-1 by KBr pellet using Perkin-Elmer 457 spectrophotometer at CDRI, Lucknow. The molar conductance was measured with digital conductivity meter (HPG system G-3001) using a Digital conductivity bridge in DMF and DMSO at 10-3 M dilution at room temperature. Magnetic susceptibility was determined by Gouy’s balance [13] using CuSO4.5H2O as calibrant. The electronic spectra of the complexes were recorded by Beckmann-DU-Spectrophotometer (Fullerton, California 92834-3100, USA). The metal and chloride contents were determined through gravimetric estimation method. The elemental analysis was carried out at CDRI, Lucknow.
Preparation of Schiff Base Ligand
2-aminophenol (1.09 g, 10 mmol) was added to the solution of 1-phenyl-2, 3-dimethyl-4-(4-iminopentan-2-one)-pyrazol-5-one (2.85 g, 10 mmol) in 50 ml of ethanol. The reaction mixture was refluxed for 10 h. On cooling, the orange solid was filtered and recrystallized from ethanol in 90% yield. The purity of the sample was checked by TLC. The ligand was characterized by the determination of melting point (146°C), elemental analysis and IR spectra.
Preparation of Transition Metal Complexes
The solution of the metal salt was added drop wise to the solution of the ligand 3 with constant stirring till the complete precipitation occurred. The precipitation was filtered, washed. The obtained product was washed with ether and dried over vacuum desiccator. The yields of the purified complexes are varying from 55-70%. The reaction scheme is shown in Figure 1 and analytical and physical data are shown in Table 1.
Table 1: Analytical and physical parameters of ligands and complexes
| S. No. | Complexes | Colour |
M.P |
Elemental analysis |
Magnetic moment (B.M.) |
Electrolytic nature |
|||||
|
0C |
% of C |
% of H |
% of N |
% of Cl |
% of M |
DMSO |
DMF |
||||
| 1 | Ligand, 3 (C22 H24N4O2)(MW= 376) | Orange |
146 |
69.86 (70.19) |
6.32 (6.43) |
14.73 (14.88) |
– |
– |
– |
– |
– |
| 2. | [TiL. 2H2O] Cl, 4(MW=493.8) | Yellow |
172 |
53.32 (53.51) |
5.10 (5.31) |
11.22 (11.35) |
7.00 (7.18) |
9.56 (9.69) |
1.76 |
1:1 |
1:1 |
| 3. | [Mn L. 2H2O] Cl, 5(MW=500.8) | Brown |
181 |
52.73 (52.76) |
5.19 (5.23) |
11.10 (11.19) |
6.98 (7.08) |
10.92 (10.97) |
4.90 |
1:1 |
1:1 |
| 4. | [VL. 2H2O] Cl, 6(MW=496.9) | Orangish yellow |
185 |
53.21 (53.18) |
5.18 (5.27) |
11.20 (11.28) |
6.96 (7.14) |
10.00 (10.25) |
2.92 |
1:1 |
1:1 |
| 5. | [RuL. 2H2O] Cl, 7(MW=547) | Green |
215 |
48.18 (48.31) |
4.72 (4.79) |
10.10 (10.24) |
6.40 (6.48) |
18.49 (18.48) |
2.10 |
1:1 |
1:1 |
| 6. | [MoOL. H2O] Cl, 8(MW=539.9) | Yellow |
209 |
49.10 (48.94) |
4.41 (4.48) |
10.28 (10.38) |
6.48 (6.57) |
17.80 (17.77) |
1.70 |
1:1 |
1:1 |
| 7. | [MoO2L], 9(MW=502.4) | White |
213 |
52.23 (52.60) |
4.10 (4.41) |
11.08 (11.15) |
– |
18.79 (19.10) |
Diamag- netic |
Non-electrolyte |
|
| 8. | [UO2 L], 10(MW=644.5) | White |
231 |
40.72 (41.00) |
3.18 (3.44) |
8.59 (8.69) |
– |
36.71 (36.93) |
Diamag- netic |
Non-electrolyte |
|
Antibacterial Activity
Antibacterial activity was tested against Gram +ve (Staphylococcus aureus and Bacillus subtilis) by the disc diffusion method using agar nutrient as medium and utilizing silver nitrate as control. The disc susceptibility test [14-16] is a relatively robust and simple technique for determining the sensitivity profiles of Staphylococcus aureus and Bacillus subtilis. However, many factors such as agar source, inoculum size, disc potency, incubation temperature and length of incubation can influence zone size diameter [17, 18]. Plate inoculation methods have included streaking and a combination of both streaking and flooding. In this study both methods of plate flooding and streaking gave reproducible results within each technique and were equally practical. The stock solutions were prepared in acetonitrile for each of the compound and all blank discs were moisturized with the solvent. Paper containing the compounds were placed on the surface of the nutrient agar plates previously spread (streaking and flooding) with 0.1 ml of overnight cultures of micro-organisms. After 36 h of incubation at 37oC, the diameter of inhibition zones was measured. The % activity Index for the complex was calculated by the formula as below:
![]()
Results and Discussion
The analytical data suggested 1:1 (M:L) stoichiometry for all the synthesized complexes as shown in Table 1. The molar conductance measurement of the complexes suggested 1:1 electrolytic nature for Ti (III), V (III), Mn (III), Ru (III), MoO(V) complexes and non-electrolytic nature for MoO2 (VI) and UO2 (VI) complexes. The magnetic moment values at room temperature are consistent with that of octahedral geometry around the central metal ion (Table 1). The MoO2 (VI) and UO2 (VI) complexes are diamagnetic in nature while all other complexes are paramagnetic in character. Thermal analysis shows that Ti (III), Mn (III), V (III), Ru (III) complexes possess two coordinated water molecules whereas MoO (V) complex has only one coordinated water molecule.
IR Spectra
The IR spectrum of the ligand was compared with those of corresponding metal complexes in order to find out the possible coordination sites. The ligand shows a weak broad band at 2950 cm-1 which has been assigned to intramolecular hydrogen bonding between enolizable -C = O of pentan-2, 4-dione and phenolic group [19]. This band has disappeared in all the metal complexes, which indicates the deprotonation of enolic and phenolic groups upon complexation. In addition to that, the absence of a band at 1660-1700 cm-1 (which is characteristic of free -C = O group) suggests that the carbonyl group is in enolic form. This has suggested dibasic nature of the ligand. The ligand shows a band at 1610 cm-1 characteristic of -C = N (azomethine group). This band is shifted to a lower frequency by 20-25 cm-1 in all the metal complexes. This indicates the involvement of nitrogen atom of azomethine group in coordination with the metal ion [20]. These coordination sites are further confirmed by the presence of non-ligand bands in the far IR region (430-490 cm-1 and 360-410 cm-1) of the complex due to VM-O and VM-N respectively [21]. All complexes, except MoO2 (VI) and UO2 (VI) show broad band in the region of 3500-5100cm-1 due to VO-H of water molecule. The coordinated nature of the water molecule/molecules is confirmed by two other non-ligand bands in the region of 840-855 cm-1 (wagging) and 740-750 cm-1 (rocking) modes of water molecules. TGA also supported the coordinated nature of water molecules [22]. The MoO (V) complex shows another non-ligand band at 950 cm-1 assignable to MO=O stretching frequency. The MoO2 (VI) complex shows bands at 960 and 910 cm-1 attributable to Vsym (O=MO=O) and Vsym (O=MO=O) respectively of Cis MoO2 configuration [23]. The UO2 (VI) complex shows Vas (OUO) and Vs (OUO) modes at 885 and 790 cm-1 respectively, suggesting trans nature (O=U=O) of UO2 group [24].
Reflectance Spectra and Magnetic Measurements
The electronic spectra of the metal complexes also supported the octahedral geometry for all these complexes. The Ti (III) complex shows a broad band at 20110 cm-1 assign to 2T2g→ 2Eg transition for octahedral symmetry [25]. The Mn (III) complex shows three bands at 19000 cm-1, 13000 cm-1 and 20,000 cm-1 assign to 5B1g → 5B2g, 5B1g →5Eg and Mn (dp) → p* (azomethine) respectively. These bands are characteristic of octahedral geometry [26]. The Ru (III) complex demonstrates three band in its electronic spectrum at 13650 cm-1, 17600 cm-1 and 22500 cm-1, which may be assigned to 2T2g→4T1g, 2T2g → 4T2g and 2T2g → 2A2g, 2T1g respectively for octahedral symmetry [27, 28]. The V (III) complex shows a band at 16000 cm-1 with shoulder at 21000 cm-1. The low energy has been assigned to 3T1g → 3T2g whereas high energy band to 3T1g → 3T1g (P) transitions, characteristic of octahedral geometry around V (III) ion [29]. The electronic spectrum of MoO (V) complex exhibits bands at 14200 cm-1, 19610 cm-1 and 25000 cm-1 assignable to 2B2→2E (dxy→dxz, dyz), 2B2→2A (dxy→dx2-y2) and 2B2 → 2A2 (dxy →dz2) transitions respectively and suggest octahedral environment around MO (V) ion [30]. The electronic spectra of MoO2 (VI) and UO2 (VI) complexes show only charge–transfer transitions.
Antimicrobial Activity
The antibacterial activity of tetradentated aromatic Schiff base ligands and their metal complexes were screened against microorganism. The microorganisms used in the present investigations include bacteria: Staphylococcus aureus and Bacillus subtilis. Minimum inhibitory concentrations (MICs) method was used to determine the antibacterial activity of the synthesized compounds. The diffusion method is very simple, it requires moistened disks with the solution of Ligand metal complex, the medium used is Mueller-Hinton agar with 2% of glucose and the diameter of inhibition zone is visually read at 36 hours after incubation at 37°C. The antibacterial activity was estimated on the basis of the size of the inhibition zone formed around the paper disks on the seeded agar plates (Figure 1).
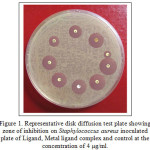 |
Figure 1: Representative disk diffusion test plate showing zone of inhibition on Staphylococcus aureus inoculated plate of Ligand, Metal ligand complex and control at the concentration of 4 µg/ml. |
The abbreviations Control (Ag NO3), 11, Ligand, 3, [TiL. 2H2O] Cl, 4, [Mn L. 2H2O] Cl, 5, [VL. 2H2O] Cl, 6, [RuL. 2H2O] Cl, 7, [MoOL. H2O] Cl, 8, [MoO2L], 9, [UO2 L], 10.
The results are presented in Table 2. A comparative study of the ligands and their metal complexes [31, 32] indicates that most of the metal chelates exhibit higher antimicrobial activity than that of the free ligand and the control as shown in Table 2. This increased antimicrobial activity of the metal chelates can be explained on the basis of overtone’s concept [33] and chelation theory [34]. The biological activity of the complexes follows the order: MoO (V) ≈ MoO2 (VI) > Mn (III) > Ru (III) ≈ UO2 (VI) >Ti (III). Furthermore, the data shows that Staphylococcus aureus was inhibited to a greater degree by the MoO (V) and MoO2 (VI) complexes, whereas Bacillus subtilis was inhibited to a greater degree by Mn and MoO. In conclusion the complexes prepared with the new Schiff base could be used for the treatment of some common diseases caused by Staphylococcus aureus and Bacillus subtilis.
Table 2: The antibacterial activity of the ligand and its metal complexes (The data shown is mean value of the three independent experiments)
| Compound |
S. aureus |
B. subtilis |
| Control (Ag NO3), 11 |
+ |
++ |
| Ligand, 3 |
+ |
+ |
| [Ti L. 2H2O] Cl, 4 |
++ |
++ |
| [VL. 2H2O] Cl, 5 |
+++ |
++ |
| [MnL.2H2O] Cl, 6 |
+++ |
++++ |
| [RuL.2H2O] Cl, 7 |
+++ |
+++ |
| [MoO.L.H2O] Cl, 8 |
++++ |
++++ |
| [MoO2L], 9 |
++++ |
+++ |
| [UO2L], 10 |
+++ |
+++ |
C* = 4 µg/ml. Inhibition zone diameter mm (% inhibition) “+, 7-11 (32-50 %), ++11-15 (50-68 %), +++ 15-19 (68-86 %), ++++19-23 (86-100 %)
Conclusion
Ti (III), Mn (III), V (III), Ru (III) and MoO (V) complexes of the Schiff base derived from 1-phenyl-2,3-dimethyl-4-(4-iminopentan-2-one)-pyrazole-5-one and 2-aminophenol and complexes are electrolytic in nature, whereas MoO2 (VI) and UO2 (VI) complexes are non-electrolytic in nature. Schiff base behaves as a neutral tetradentate ligand and is coordinated to the central metal ion. On the basis of studies performed octahedral geometry has been proposed for all the synthesized complexes. The biological activity of all the complexes is higher than free Schiff base ligand and follows the order: MoO (V) ≈ MoO2 (VI) > Mn (III) > Ru (III) ≈ UO2 (VI) > Ti (III). This means that metal chelation significantly affects the antimicrobial behavior of the organic ligand.
References
- Krishnankutty, K. and Ummathur, M. B. J. Indian Chem. Soc. 83: 663(2006).
- Schiff, H., Synthesis of Sciiff Bases. Ann. Suppl. 3: 343(1864).
- Singh, B.K. and Adhikari, D. Intern. J. Basic Appl. Chem. Sci. 2: 84-107(2012).
- Khandar, A.A., Hosseini-Yazdi, S.A., Zarei, S.A and Rabie, U.M. Inorg. Chim. Acta. 358: 3211(2005).
- Manikshete, A.H., Sarsamkar, S.K., Deodware, S.A., Kamble V.N. and Asabe, M.R. Inorg. Chem. Commun. 14: 618–621(2011).
- Yamada, S. Coord. Chem. Rev. 190: 537(1999).
- Cimernan, Z., Galic, N. and Bosner, B. Anal. Chim. Acta. 343: 145(1997).
- Panniyamurthy, T., Bhatia, B., Reddy, M.M., Maikap G.C. and Iqbal, J.Tetrahedron. 53: 7649(1997).
- Refat, M.S., Killa, H.M.A., Mansour, A.F. and Fetooh, H., Synth. React. Inorg. Metal-Org.Nano-Met. Chemist. 41: 295-308(2011).
- Saritha, P., Reddy, B., Satyanarayan and Jayatyagaraju, J. Indian Chem. Soc. 83: 1204(2006).
- Mohamed, G.G., Omar, M.M. and Hindy, A.M. Turkish J. Chem. 30: 361-382(2006).
- Phaniband, M.A. and Dhumwad, S.D.Trans. Met. Chem. 32: 1117(2007).
- Saunderson, A. Phy. Edu. (1968) 3: 272–273.
- Durairaja, S., Srinivasan, S. and Perumalsamy, P.L. Electron. J. Biol. 5: 5(2009).
- Bauer, A., Kirby, W., Sherris, J.C. and Turck, M. American J. Clin. patho. 45 (4): 493(1966).
- Salmon, S.A., Watts, J.L., Case, C.A., Hoffman, L.J., Wegener, H.C. and Yancey, R., J. Clin. Microbiol., 33: 2435-2444(1995).
- Acar, J.F. and Goldstein, F.W. Antibiotics in Laboratory Medicine. In: V. Lorian, Williams and Wilkins, Baltimore, MD, pp.27-63(1986).
- Goldstein, F., Chumpitaz, J., Guevara, J., Papadopoulou, B., Acar, J. and Vieu, J., J. Infect. Dis. 153: 261-265(1986).
- Raman, N., Kulandaisamy, A. and Jeyasubramanian, K. Indian J. Chem. 41A: 942-949(2002).
- Thankamony, M.and Mohanan, K. Indian J. Chem. 46: 249(2007).
- Nakamoto, K. Infra red and Raman spectra of inorganic and coordination compounds, 3rd Edn., Wiley Inter science, New York, pp 85-88(1977).
- Kriza, A., Reiss, A., Florea, S. and Caproiu, T. J. Indian Chem. Soc. 77: 207(2000).
- Singh N.K.and Singh, S.B. Indian J. Chem. 40: 1070-1075(2001).
- Syamal A.and Maurya, M.R. Indian J. of Chem. 24A: 836-840(1985).
- Gupta, S., Roy, S., Mandal, T.N., Das, K., Ray, S., Butcher, R.J. and Kar, S.K. J. Chem. Sci. 12: 239-245 (2012).
- Rastogi, R.K., Garg, P. and Ahmad, S. Asian J. Chem. 24: 1043-1048 (2009).
- Singh, M.K., Singh, A.K., Gupta, P.K., Jaipal and Sharma, L.K. Indian J. Chem. 41: 1385-1390 (2002).
- Mishra, L. and Sinha R. Indian J. Chem. 29: 1131(2000).
- Thangadurai, T. D. and Natrajan, K. Indian J. Chem41A: 741-745 (2002).
- Syamal, A. and Singh, M.M. Indian J. Chem. 32A: 42-48 (1993).
- Gupta, Y.K., Agarwal, S.C., Madnawat, S.P., Narain, R. Res. J. Chem. Sci. 2: 68-71 (2012).
- Patel, K.B., Patel, R.B., Vyas, K.B. and Nimavat, K.S. Der Pharma. Sinica. 3: 501-506 (2012).
- Aryancyula, Y. and Rao, R.P. Synth. React. Inorg. Met-Org. Chem. 26: 257 (1986).
- Misra, L. and Singh, V.K. Indian J. Chem. 32A: 446 (1993).

This work is licensed under a Creative Commons Attribution 4.0 International License.









