Synthesis and Structure Elucidation of Cr(III) Complexes of Polydentate Hydroxamic Acid Ligands
K. P. Srivastava* and Ali Akbar1
2Ganga Singh College, J.P.University, Chapra - 841 301, India.
1Department of Chemistry, K.K.College of Engineering and Management, Biharsharif, Nalanda, Bihar, India.
Corresponding Author E-mail: gscdrkpsritprin@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/290244
The ligands α-mercaptobenzacetohydroxamic acid (MBAHA-H) and 2-amino-α-mercapto-benzacetohydroxamic acid (AMBAHA-H) and their different mixed ligand novel complexes with CrIII having specific formulae have been synthesized and characterised by elemental analyses, magnetic and conductance measurements, IR and electronic spectral studies. The ligands were found to behave as monobasic tridentate (SO’O donor) and tetradentate (SO’ ON donor) manner respectively. All the synthesized CrIII complexes were non-electrolyte with magnetic moment ranging from 3.79 to 3.87 BM. The structural assessment of the complexes has been carried out based on spectral studies (electronic&infrared) and molar conductivity values. All the complexes were found to be of octahedral geometry.
KEYWORDS:Hydroxamic acids; polydentate ligands; octahedral complexes; magnetic moment
Download this article as:| Copy the following to cite this article: Srivastava K. P, Akbar A. Synthesis and Structure Elucidation of Cr(III) Complexes of Polydentate Hydroxamic Acid Ligands. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Srivastava K. P, Akbar A. Synthesis and Structure Elucidation of Cr(III) Complexes of Polydentate Hydroxamic Acid Ligands. Orient J Chem 2013;29(2). Available from: http://www.orientjchem.org/?p=22215 |
Introduction
Hydroxamic acids are versatile reagents for organic and inorganic analyses[1,2]. Their derivatives are biochemically highly active and find applications in medicinal use[3]. The sulphur containing derivatives of hydroxamic acid have aroused considerable interests over decades[4]. Despite the ligational potentiality associated with sulphur derivatives of hydroxamic acid, the studies made on the metal complexes of these ligands are limited[5]. The study of coordination compounds of sulphur donor ligands is of biochemical interests due to their carcinostatic (anticancer)[6] antibacterial[7] and antifungal[8] activities. Sulphur donor compounds are expected to be the most effective anticancer agents as they usually confer lipid solubility on the metal complex. The sulphur donor ligands have also been widely used as agro-chemicals and favorable environmental degradation. In continuation[9-13] of our work on the complexes with the mentioned ligands, we report here the synthesis and structural characterization of two new ligands, 2-amino-α-mercaptobenzacetohydroxamic acid (AMBAHA-H) and α-mercapto benzacetohydroxamic acid, (MBAHA-H) (Figure-1) and their complexes with Cr (III).
Experimental
Materials
All the chemicals and reagents used were of Analar grade. Anhydrous grade alcohols, DMF, and DMSO were obtained from Fischer scientific. 2-aminobenzhydroxamic acid was obtained from Aldrich (USA). The metal chlorides/acetates used were of BDH AR grade in the present investigation. All reactions and experimental manipulations were carried out at appropriate temperature.
Physico chemical measurements
Elemental analysis (C, H, S& N) of ligands and complexes was carried out in micro analytical laboratory on Carl-Ebra 1106 elemental analyzer. Metal in the complexes was estimated following standard procedure [14]. The molar conductance measurements were carried out for the 10-3M solutions of complexes in DMSOsolvent at 300K using a Systronics direct reading digitalConductivity Bridge-304 with a dip type cell. The magnetic measurements of the complexes at 300K were made by Gouy magnetic balance using Hg [Co (NCS)4] as calibrant. The measured susceptibilities were corrected for diamagnetic susceptibility of the ligand. The IR spectra of ligand and the complexes as nujal-mull smears were recorded in the region 4000-200 cm-1 on a Perkin-Elmer 577 spectrophotometer. The electronic spectra of the complexes were recorded on Systronics UV-Visible spectrophotometer Type –119 PC based (λ= 200-1000 nm & band width 2 nm) using ethanol as the solvent.
Synthesis of Ligand [MBAHA-H = L-H]
The ligand MPA-H was prepared the method reported by Y. Inoue & H.Yukawa [15]. One mole of KOH (56.10 g) dissolved in 140 ml of methanol was added to a solution of 0.67 moles of hydroxylamine hydrochloride (46.7 g) in methanol. Both solutions were mixed together keeping the temperature range at 35-400C.The mixture was left in ice-bath for five minutes ensuring the complete precipitation of KCl. Then 0.35 moles of α-mercaptophenyl acetate was added in portions constant shaking and after the addition is complete the solution was filtered immediately through suction. The residue in funnel was washed with little methanol. The filtrate was kept in Erlenmeyer flask for 48 hours. Crystals of potassium salt of the acid were filtered, washed with a little absolute alcohol and dried in air.
About half of the yield was mixed with 80 ml 1.25N acetic acid and stirred while heating until a clear solution was obtained. The solution was allowed to cool at room temperature and finally chilled in ice-bath. The ligand, MBAHA-H, was separated out as light brown crystals. The melting point (decomposition point) was recorded and found to be 186-1880C.The stoichiometry of the synthesis reaction is represented in the form of equation as:
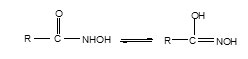
Synthesis of Ligand [AMBAHA-H = L’H]:
This was also prepared by the method described above. The alkaline solution of hydroxylamine was prepared as above mixing the methanolic solutions of KOH and hydroxylamine hydrochloride. The solution of 0.175 mole of 2-amino α-mercaptophenyl acetate in methanol was added to it to get the potassium salt of the acid. The free acid was obtained by treating the potassium salt with acetic acid. Yellowish brown crystals ofthe ligand were obtained. The melting point (decomposition point) was recorded and found to be 198-1990C.
The formation of ligand can be represented by the following chemical equation:
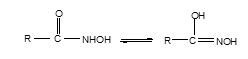
Synthesis of Complexes:
(A) Synthesis of Complexes of Type [MLX.H2O] & [ML’X]Where, X = Cl.
About 0.041 moles of metal chloride were dissolved in 40 ml of distilled water and added to an aqueous ethanolic solution of the ligand (0.40 moles in 35 ml water) slowly with constant shaking. The different coloured heavy precipitate was separated out. It was filtered, washed with distilled water until free from chloride and dried at 140-1450C.
EtOH
CrCl3.6H2O + L-H ——————→ [CrLCl (H2O)2]
Light green
EtOH
CrCl3.6H2O + L’-H ———————→[CrL’Cl (H2O)]
Dark green
(B) Synthesis of Complexes of Type [MLXB2] and [ML’XB]
Where, X = Cl andB = Pyridine (-py) & Pyridine-N-oxide (-py-O)
Aqueous ethanolic metal salt solution (0.021moles in 40ml of aq. ethanol) was mixed with ethanolic solution of the ligand (0.02 moles in 50 ml) and warmed with base on water-bath. It was further digested for a few minutes and the precipitate was filtered. It was washed with water several times and then with ethanol. It was dried at room temperature. Complexes with other bases were prepared similarly. The complexes obtained were collected for analysis and characterisation.
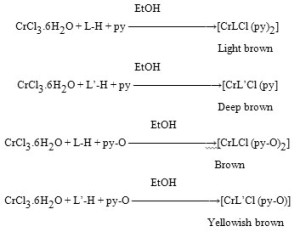
Yellowish brown
Results and Discussion
Physico-chemical characterizations and geometricalconfiguration of the complexes
Cr(III) salt reacts withMBAHA – H and AMBAHA – Hligands in 1:1 molar ratio in alcoholic medium to afford darkreddish/brown complexes. The ligand and its complexes are stable at room temperature and are non-hygroscopic. The ligandswere soluble in common polar organic solvents, such as ethanol, methanol, and chloroform but partially soluble in hexane. The complexes were relatively wellsoluble in DMF and DMSO. The synthesized ligand andits complexes were characterized by elemental analysis,spectra, and molar conductivity measurements. Thegeometry of the newly synthesized compounds has beenelucidated based on their elemental analysis, molarconductivity and spectral data.
Elemental Analysis
The stoichiometry of the ligands and their complexes were confirmed by their elemental analysis.The metal / ligand ratio was found to be 1:1 has been arrived at by estimating the metal and nitrogen and sulphur contents of the complexes. Elemental analysis of ligands and their Cr (III) complexes show good agreement with the proposed structures of the ligands and their complexes (Table-1).
Molar conductance measurements
The molar conductance values (11-23 ohm-1cm2mol-1) of the complexes which were determined in DMSO solvent indicate that the complexes under study are non-electrolytic in nature [16](Table-2).
Magnetic Susceptibility Measurements
The observed magnetic moments of Cr(III) complexes of MBAHA-H & AMBAHA-H are given in table-2. The observed values of magnetic moment for Cr(III) complexes are generally diagnostic of the coordination about the metal ion.
The magnetic measurements on the complexes reported herein show that all were paramagnetic and have three unpaired electrons indicating high-spin octahedral stereochemistry[17]. For the present complexes these values lie in the range 3.87 to 3.92 B.M. and are being independent of temperature.
IR Spectral Studies
The structural possibilities of the complexes depend upon the mode of coordination of the ligands. The IR spectral studies are quite useful on determining the mode coordination of ligands. On critically examining the position and direction of the shifts of the frequencies of the ligands in the complexes, as compared to their positions in the free state, the mode of coordination can be suggested for all the investigated complexes.
The IR spectral studies show that the ligand MBAHA-H acts as mono negative tri dentate (SOO’ donor) ligand but AMBAHA-H acts as mono negative tetra dentate (SOO’N donor) ligand. The ligand MBAHA-H coordinating with metals through thioalcoholic S, carbonyl O and hydroxamic O atoms respectively [Figure-1] andthe ligand AMBAHA-H coordinating with metals through thioalcoholic S, carbonyl O hydroxamic O atoms and primary amino N atoms respectively [Figure-2].The main infrared bands and their assignments are presented here in table-3.
Vibrations Due To MBAHA -H Moiety:
The absorption bands in the region 3280-3260 cm-1 in the free ligands are assigned to NH-stretching frequencies. The band at 3265 cm-1 due to ν(N-H) mode remained intact in the complexes indicating the non-participation of N-H of MBAHA-H group in coordination.
The infrared spectra of the ligand showed a medium intensity band at 2560 cm-1 for ν(S-H) mode. Absence if this band in the complexes indicated the destruction of the S-H bonding followed by complexation of S after deprotonation [18]. This was further supported by a downward shift in ν(C-S) by 10-20 cm-1 in the complexes and the appearance of new low intensity bands at 280-270 cm-1due to M-S stretching vibrations.The appearance of medium intensity band in the region 2700-2400 cm-1 in the infrared spectra of free ligand indicated the possibility of tautomerism as shown below:
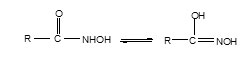
The ν(C= S) stretch shifted to lower frequency in the complexes by 45-30 cm-1, suggesting involvement of O of (C=O) in coordination. This was further supported by the appearance of low intensity bands at 490-470 cm-1 in the spectra of complexes due to the ν(M-O) stretch.
The ν(O-H) stretch shifted to lower frequency in the complexes by 25-15 cm-1 suggesting coordination of ligand through O-H group with metal cations.
It was observed the additional bands in the regions 480-435 cm-1and 355-290 cm-1in the complexes which are assigned to ν(M-O) and ν(M-S) stretching vibrational modes respectively.
Further, it was observed that ν (M-O) frequency shifted downward with increasing mass of the metals.The study and comparison of infrared spectra of the free ligand MBAHA-H and its complexes with selected common bivalent transition metals imply that the ligand behaves as a uninegative tridentate (SOO donor) ligand and the metal ion are connected through carbonyl O, hydroxamic O and thioalcoholic S atom.
Vibrations Due to AMBAHA-H Moiety
The ability to locate the various group frequencies corresponding to typical linkage in the investigated AMBAHA-Hligand and in its synthesized complexes is not so easy. Although there have been many attempts to assign empirically a few I.R. bands of metal complexes of AMBAHA-H, no complete assignments of their I.R.spectra have been available.
A fairly reliable assignment of I.R. frequencies is now possible to its complexes [19-20], though normal coordinate analysis using Wilson’s G.F. matrix method and the Urey Bradley force filed functions. A computational method based on a normal coordinate analysis and quantum chemical dipole moment calculations have been suggested for predicting transition moment directions and dichoric ratios in molecular crystals of low symmetry [22].
The infrared spectra of the ligand AMBAHA-H in free state showed a medium intensity band at 2570 cm-1for ν(S-H) vibration. Absence of this band in its complexes with investigated bivalent transition metals indicates the destruction of the S-H bonding followed by complexation of S with metal after deprotonation. This is further supported by a downward shift in ν(C-S) modes by 30-15 cm-1 in the investigated complexes and the disappearance of a new low intensity band at 300-275 cm-1 due to M-S vibrations.The band at 3275 cm-1 due to v(N-H) mode remains intact in complexes indicating the non-participation of N-H of hydroxamic acid group in coordination.
Further, the I.R.spectra of the metal complexes of AMBAHA-H ligand show the band attributed to v(NH2) of the coordinated amino group, which appeared at Δv> 50 cm-1 than in the spectrum of free ligand.
The v(C=O) stretch shifts to lower frequency in the complexes by 30-15 cm-1 suggesting involvement of carbonyl oxygen atom in coordination. This is further supported by the appearance of new low intensity bands around 500-450 cm-1 in the spectra of investigated complexes due to the v(M-O) stretch.
The v (O-H) stretch of hydroxamic group shifts to lower frequency in the investigated complexes by 30-20 cm-1suggesting coordination of AMBAHA-H through O atom of O-H group with metals. The comparison with the infrared spectra of the ligand, the investigated complexes show new bands in the far infrared regions, 500-450, 400-350 and 300-275 cm-1 assignable to M-O, M-N, and M-S stretching vibrations respectively. The above discussions clearly indicate that the ligand AMBAHA-H serves as a uninegative tetra dentate (SO’ON donor) in all the investigated complexes coordinating through the carbonyl oxygen, hydroxamic oxygen, thioalcoholic sulphur and amino nitrogen atoms withCr3+ in the investigated complexes[23].
The v(M-Cl) has been assigned in 250-215 cm-1 regions in the present studies.The infrared spectra of metal complexes with pyridine (-py) and pyridine-N-oxide (-py-O-) have been studied extensively. Upon complex formation, the pyridine vibrations in the high frequency region (650-420 cm-1) are shifted to higher frequencies (290-230 cm-1) for the investigated metal complexes. The N=O stretching band of pyridine N-oxide (1265 cm-1) is shifted by 70-30 cm-1 to a lower frequency upon complexation.The infrared spectra of investigated metal complexes exhibited characteristic bands due to coordination of water molecules around 490-310 cm-1.
Electronic Spectral Studies
The study of magnetic and electronic spectra data is quite informative in characterizing the geometry of the complexes. The UV-visible spectrum of the ligands and their complexes were recorded in DMSO solution.
For octahedral complexes of Cr (III) ion [d3 system (t2g3eg0)], the following three spin-allowed ligand field absorption bands corresponding to the transitions:
4A2g 4T2g(F) v1 ~ 19800-17400 cm-1 (10Dq)
4A2g 4T1g(F) v2~ 26300-24600 cm-1 (18Dq – x)
4A2g 4T1g(P) v3~ 36400-37800 cm-1 (15B’ + 12Dq + x)
Of these transitions v1 is independent of configuration interaction and gives a better value of 10Dq. v3transition is highest in energy because of additional rise in energy of4T1g(P) due to term interaction parameter x. The molar absorptivity of these bands is generally below 20. Some spin-forbidden triplet to singlet bands may also appear.
Accordingly, the electronic absorption spectra of the investigated complexes of CrIII reported herein exhibit three spin-allowed bands, which indicate their regular octahedral geometry (Table-4).
The ligand field parameters 10Dq and B for the investigated complexes have been computed from equations suggested by Lever. These data are in good agreement with those reported for other octahedral complexes[23].
As seen from the energy diagram, the separation between 4T2g(F) &4A2g is equal to10Dq. The spectral data for investigated complexes are listed in table-4.The ratio of frequencies v2/v1of the 2nd and 1st maxima lies in most cases between 1.5 and 1.7.It is noted from the Tanabe-Sugano diagram, for d3 system that the (t2g3eg0) and thus involves the promotion of two electrons from the t2g orbital. Since all the three bands are observed, the value of B’ for the complexes may be calculated from the eg.
15B’ = v2 + v3 – 3v1
On the basis of the forgoing evidences,the proposed octahedral geometry i.e., Oh symmetry for the complexes is presented in figure-1and 2.
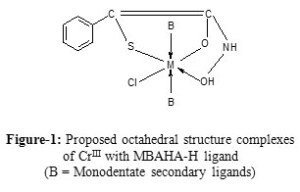 |
Figure1: Proposed octahedral structure complexes of CrIII with MBAHA-H ligand (B = Monodentate secondary ligands). |
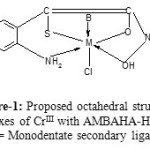 |
Figure-1: Proposed octahedral structure complexes of CrIII with AMBAHA-H ligand (B = Monodentate secondary ligands). |
Conclusions
Six complexes of the ligands MBAHA-Hand AMBAHA-Hwith Cr3+ havebeen synthesized and characterized. The IR spectral studies revealed that the ligands MBAHA-H and AMBAHA-Hacted as a mononegative tridentate (SO’O donor) and tetradentate (SO’ON donor), respectively in all the complexes. The magnetic, conductance and electronic spectral studies revealed that the complexes were paramagnetic with octahedral geometries. All the investigated complexes were non-electrolyte and monomer.
References
- Agrawal, Y. K. Russ. Chem. Rev., 48, 1773 (1979).
- Agrawal, Y. K. Rev. Anal. Chem., 4, 274 (1980).
- Prusoff, W. H. Lajtha L. G and Welch, A. D. Biochim. Biophys. Acta., 20, 209 (1956).
- Inoue Y.and Yukawa, H. J. Agr. Chem. Soc., Japan, 16, 504 (1940).
- Satpathy et al. Acta. Chim. Hung., 125, 527 (1998).
- Thaker B. T. and Patel, B. V. Thermochima. Acta., 129, 161 (1988).
- I. Nakagawa and T. Shimanouchi, Spectrochim. Acta. 20, 429 (1964).
- Lave, T. J. Nekagawa, I. Walter J. L. and Kadethil, A. K. Inorg. Chem.1, 267 (1962).
- Srivastava K. P. and Ojha, N. K. Asian J. Chem., 19(1), 385 (2007).
- Srivastava K. P. and Ojha, N. K. Orient J. Chem., 22, 441 (2006).
- SrivastavaK.P.& OjhaN.K.Int. J. Chem. Sci. 5, 3, 2248, (2008).
- Srivastava, K.P. et al.;Int. J. Chem.Tech. Res. 1,1,71, (2009).
- SrivastavaK.P. &Akbar, A.Int. J. Chem. Sci. (accepted) (2013).
- Vogel,A. I.; A Textbook of Quantitative Inorganic Analysis; 6th edn. Wiley NY (1996).
- Inoue Y.& Yukawa,H.;J.Agr.Chem. Soc. (Japan) 16, 604, (1940).
- Livingnstone, S. L. Inorg. Chem. Acta.5, 119 (1971).
- Geary, W. J. Coord. Chem. Rev., 7, 81 (1971).
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, John Wiley and Sons, NY (1997).
- Kulaweic, R.J. &Crabtree, R.H. Coord. Chem. Rev. 99,89 (1990).
- Kofod, P.Inorg.Chem. 34,2768 (1990).
- Zerbi, G.& Alberti, C.Specrochim.Acta. 30, 1261 (1977).
- West, D.X.et al. Transition Met. Chem. 19, 195(1994).
- Akbar Ali, Ph.D. Thesis, J.P.University, Chapra, Bihar (2011).

This work is licensed under a Creative Commons Attribution 4.0 International License.









