Study of Antioxidant Activity of Spinacia oleracea L.
Farhad Hatamjafari and Maryam Tazar v*
Department of Chemistry, Faculty of Science, Tonekabon Branch, Islamic Azad University, Tonekabon, Iran. Corresponding Author E-mail: Maryam.tazarv@yahoo.com
The Methanol extract of Iranian Spinacia oleraceae L between Babol and Varamin regions was examined. In addition, total amount of DPPH (1,1-diphenyl-2-picryl hydrazyl) radical scavenging activities and reductive power of crude extracted its different fractions were determined. This research has shown Spinach has antioxidant activity.
KEYWORDS:Antioxidant activity; Spinach; DPPH
Download this article as:| Copy the following to cite this article: Hatamjafari F, Tazar V. M. Study of Antioxidant Activity of Spinacia oleracea L. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Hatamjafari F, Tazar V. M. Study of Antioxidant Activity of Spinacia oleracea L. Orient J Chem 2013;29(2). Available from: http://www.orientjchem.org/?p=22056 |
Materials and Methods
PlantmaterialandExtraction
Spanich with scientific term Spinacea oleraceal is one of important vegetable of beet group .It is mentioned that in central regions of Asia and also Iran that it is planted more than 2000 years1-4. Spanich is one of the most important vegetable that it have important nutritious values and its leafs and stems are used as it has second rank between 42 kinds of fruit and vegetables because of 10 kinds of vitamins and mineral matters and is high score of mineral matters and proteins, specially ascorbic acid (vitamin C)5. Spanich is rich in calcium and iron that calcium is as oxalate calcium and unavailable. Also, oxalic acid combined with magnesium and iron and cause it unavailable6. Weak availability of calcium from Spanich in human and rabbit has been provet7. Amount of oxalic acid exist in spanich leaf, 658-1670 mg in 100 g has been reported. Ascorbic acid and glycolate are materials cause produce oxalic acid in Spanich leaf. Spanic leaf have 3/2 percent of protein and its leaf protein is reduction factor in cholesterol. Spanich leaf have 6 percent of fat and linolenic acid (ω-3) and linoleic acid (ω-6) are most important fatty acids in this plant. Amount of fiber existin Spaich leaf is 65 percent. Spanich is rich in antioxidant such as betacaroten and lutein. These have antioxidant properly in and cancer. Betacaroten cause improvement in lung surgery, reduction in diabetes risk and lutein cause reduction in cataract risk and improvement in sign in old age8-9. The present study aimed to evaluate the antioxidant (DPPH) propertyof the Spinach collected from Varamin and Babol regions in Iran.
Materials and Methods
Plant material and Extraction
Spinach were collected in the autumn of 2012 in Babol and Varamin regions, Iran, from the branches exposed to sunlight. Spinach were cut after washing into small pieces and dried in the shade (30 °C) for a week. Dried Spinach were ground into a fine powder using a homogenizer. A hydroalcoholic extract of the Spinach was prepared by the maceration method, using a ratio of 1 g of Spinach to 5 mL of MeOH (99%) for 48 h. Flasks were shaken several times. The extract was filtered and the solvent was evaporated using a rotary evaporator. The extract was then dried at 40 °C and powdered10-11.
Antioxidant activity DPPH assay
The hydrogen atoms or electrons donation ability of the corresponding extracts and some pure compounds were measured by bleaching the purple colored methanolic solution of DPPH. The effects of methanolic extract and essential oil on DPPH radicals were evaluated according to a method described elsewhere. 4 mL samples of various concentrations of the extracts in methanol were separately added to a 1 mL solution of DPPH radical in methanol (final concentration of DPPH was 0.2 mM). The mixture was shaken vigorously and allowed to stand for 30 min after which the absorbance of the resulting solution was measured at 517 nm with a spectrophotometer (Rayleigh UV-1601). Inhibition of free radical DPPH as percentage [I(%)] was calculated as follows:
I (%) = 100 × (Ablank -A sample) / Ablank
Where Ablank is the absorbance of the control (containing all reagents except the test compound) and A sample is the absorbance of the test compound. IC50 value (μg /mL) is the effective concentration at which DPPH radicals are scavenged by 50%. This was obtained by interpolation and using linear regression analysis12.
DPPH determination
10 grams of powder was weighted and then mixed in 50 ml of methanol and then extracted for 24 hours in a lab temperature to get put, then the mixture is passed through the filter paper we put it in centrifuge for 20 minutes to obtain a concentrated extract (rpm 4000). After gaining weight again extracted with methanol extract (99%) prepare 100 ml the volume to make the mother solution. Mother solutions of different concentrations of extract, 100, 150, 200 and 250 mg/ml were used. To measure the antioxidant activity of the solution from Brand-Wiliames using DPPH, mg/ml were used12.
A small amount of the solution was poured into test tubes, each tube mother 10 ml of methanol added, Then 0.1 ml of each tube was removed and the other test tube, and it amounted to 3.9 ml DPPH solution were added. This was repeated three times, and 12 were measured for each concentration of test tubes 3 tube will. Test tubes were placed in the dark for 30 minutes if you have activity antioxidant change from purple to yellow color of antioxidant samples will be dissolved after its absorption at 517 nm by spectrophotometer is read.
Formula Antioxidant activity:
IC50 = 100 × (A-B / A)
Concentration of the extract, that take 50% of the trap free radicals = IC50
The absorbance of the control (DPPH and solvent-free extraction of all such factors) = A
The absorption of tested extract = B
It is seen in 0.5 ml of test solution +2.5 ml methanol solution of DPPH
Blank samples was methanol solution.
Table 1: Tables of Absorbance Concentrations of Extract, 100, 150, 200 and 250 Mg/Ml in Babol Region
Test 1
|
No. |
Conc(mg/ml) |
Absorbance |
|
1 |
100 |
2.667 |
|
2 |
150 |
2.666 |
|
3 |
200 |
2.654 |
|
4 |
250 |
2.656 |
|
Blank |
|
2.678 |
Test 2
|
No. |
Conc(mg/ml) |
Absorbance |
|
1 |
100 |
2.645 |
|
2 |
150 |
2.65 |
|
3 |
200 |
2.647 |
|
4 |
250 |
2.648 |
|
Blank |
|
2.679 |
Test 3
|
No. |
Conc(mg/ml) |
Absorbance |
|
1 |
100 |
2.658 |
|
2 |
150 |
2.651 |
|
3 |
200 |
2.668 |
|
4 |
250 |
2.631 |
|
Blank |
|
2.681 |
Table 2: Tables of Absorbance Concentrations of Extract, 100, 150, 200 and 250 Mg/Ml in Varamin Region
Test 1
|
No. |
Conc(mg/ml) |
Absorbance |
|
1 |
100 |
2.635 |
|
2 |
150 |
2.657 |
|
3 |
200 |
2.663 |
|
4 |
250 |
2.659 |
|
Blank |
|
2.679 |
Test 2
|
No. |
Conc(mg/ml) |
Absorbance |
|
1 |
100 |
2.678 |
|
2 |
150 |
2.664 |
|
3 |
200 |
2.661 |
|
4 |
250 |
2.663 |
|
Blank |
|
2.678 |
Test 3
|
No. |
Conc(mg/ml) |
Absorbance |
|
1 |
100 |
2.661 |
|
2 |
150 |
2.657 |
|
3 |
200 |
2.65 |
|
4 |
250 |
2.653 |
|
Blank |
|
2.682 |
Resultsand Discussion
Antioxidant DPPH assay results
To determine the antioxidant activity of the plant on Spinach, Varamin and Babol regions with different concentrations were evaluated. In other words, in this experiment, both the pecan harvest (two levels) and concentration (4 levels) and the interaction of those factors was studied. Moreover, the analysis of variance(ANOVA) showed that different concentrations of the extract and concentration, as well as a significant effect on the interaction of free radicals had set a trap. We used the Duncan for treated groups to identify the concentration levels studied, multiple test. The result of this test is given in the following table.
Table 3: Anova Table for Percent Inhibition of DPPH Free Radical
|
Sources |
sum of squares |
Degree of freedom |
Mean square |
F |
significantly |
|
|
ratio |
||||||
|
Collection region |
60.611 |
1 |
60.611 |
10.491** |
0.005 |
|
|
Concentration of extract |
129.591 |
3 |
43.197 |
7.477** |
0.002 |
|
|
Collection region* Concentration of extract |
7.975 |
3 |
2.658 |
0.46 |
0.714 |
|
|
Error |
92.441 |
16 |
5.778 |
|
|
|
|
Total |
2438.416 |
24 |
|
|
|
|
|
Corrected Total |
290.618 |
23 |
|
|
|
|
|
a. R Squared = 0.682 (Adjusted R Squared = 0.543) |
||||||
* significantly at 5% – ** significant at 1% – no stars is not significant
Analysis of variance also showed that different concentrations of the extract has a significant effect on the percentage of free radical trap set at 1% and probably 99% (P <0.01). The interaction between those factors did not have significant effects on free radical scavenging.
treated groups to identify the concentration levels studied, the Duncan test was used for multiple The result of this test is given in the table below.
Table 4: Comparison Of Mean Inhibition Percentage Of Free Radical DPPH with Different Concentrations of the Extract of Spinach
|
Concentration of extract |
N |
Subset |
||
|
1 |
2 |
3 |
||
|
100 |
6 |
6.6233 |
|
|
|
150 |
6 |
8.0017 |
8.0017 |
|
|
200 |
6 |
|
10.5583 |
10.5583 |
|
250 |
6 |
|
|
12.6567 |
|
Sig. |
|
0.335 |
0.084 |
0.15 |
|
Means for groups in homogeneous subsets are displayed. |
||||
|
Based on observed means. |
||||
|
The error term is Mean Square(Error) = 5.778 |
||||
|
a. Uses Harmonic Mean Sample Size = 6.000 |
||||
|
b. Alpha = 0.05 |
||||
|
|
||||
*significantly at 5% – ** significant at 1% – No stars is not significant
Duncan test results and comparison of the data showed that with increasing concentration of plant extract inhibited DPPH free radicals increases. The concentration of 100, 200 and 250 mg / ml showed significant differences with each other. Concentration of 250 mg / ml and 150 mg / ml were significantly different from each other. Column chart below (1) show the trap of free radical to dispose of any of these concentrations.
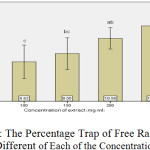 |
Graph 1: The Percentage Trap of Free Radicals in Different of Each of the Concentration: Click here to View graph |
The following graph (1), the percentage trap of free radicals associated with each of these concentrations to separate the two species have been named Babylon and spinach.
As can be seen in the graph (2) below, the percentage is much higher than that trap free radicals in Babol Spinach more than Spinach Varamin This chart shows that the highest concentration for 250 mg / ml to trap free radicals in plant extracts of spinach caught up in Babol, and the lowest concentration for 100 mg / ml plant extracts was from Spinach Varamin. Different concentrations of plant extract Spinach Varamin in relation to trap free radicals have done the same.
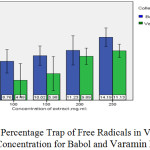 |
Graph 2: The Percentage Trap of Free Radicals in Various of Each of the Concentration for Babol And Varamin Regions: Click here to View grap |
As can be seen in the above chart, the percentage trap of free radicals for spinach is in Babol more than Varamin. This chart tells to us that highest concentration of 250 mg / ml to trap of free radicals in spinach plant extracts Babol and the lowest level of the trap concentration is 100 mg / ml Varamin region.
Conclusion
The evaluation of the antioxidant activity by DPPH test has indicated that the extract of Spinach had accepteble antioxidant activity as this study showed most caught up in the free radical concentration was 250 mg/ml Babol region and also set the lowest trap free radicals was 100 mg/ml for Varamin region.
Acknowledgements
We gratefully acknowledge the financial support from the Research Council of Tonekabon Branch Islamic Azad University.
References
- Kallo G and Bergh B O., Genetic Improvement of Vegetable Crop. Percamon Press., 833 (1993).
- Kawazu Okimura Y M., Ishii T and Yui S., Scientia Horticulturae., 97: 203 (2003).
- Salunkhe D K and Kadam S S., Marcel Dekker, INC., 721 (1998).
- Rubatzky V E and Yamaguchi M., Chapman and Hall., 843 (1997).
- Salunkhe D K., Bilon H R and Reddy N R., CRC Press, Boca Raton., 1: 285 (1991).
- Singh V., Pande P C and Jain D K., Rastogi Publications, India., (1997)
- Nolte J., Wiley- VCH Verlag GmbH and Co. KgaA., 206 (2003).
- Singh R P., Murthy K N C and Jayaprakasha G K., Journal of Agriculural and Food Chemistry., 50: 81(2002).
- Metcalf L C., Shmitz P. A and Pelca J R., Analytical Chemistry., 38: 514 (1996).
- Holland B., Unwin I D and Buss D H., The composition of foods, HM SO, London., (1991).
- Simopoulos A P., Prostaglandins, Leukotrienes and Essential Fatty Acids., 60: 421 (1999).
- Brand-Williams W., Cuvelier M and Berset C., LWT-Food Sci Technol., 28: 25 (1995).

This work is licensed under a Creative Commons Attribution 4.0 International License.









