Simultaneous Voltammetric Determination of Vitamine B9 and B12 using a Hydroquinone Derivative Multi-Wall Carbon Nanotubes Paste Electrode
M. Reza Shishehbore
Faculty Member of Basic Science, Department of Chemistry, Islamic Azad University, Yazd Branch, Iran.
Corresponding Author E-mail: shishehbor47@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/290229
In this study, the electro-oxidation of vitamin B9 and vitamin B12 and their mixture has been studied by modified multi-wall carbon nanotubes paste electrode of a synthetic hydroquinone derivative using cyclic voltammetry, chronoamperometry and differential pulse voltammetry. The cyclic voltammetric responses of the modified electrode showed the characteristic shape typical of an ECcat process. The obtained catalytic peak current by differential pulse voltammetry was linearly dependent on the vitamin B9 on concentration range 1.5-135.3 μM with limit of detection 0.2 μM. The proposed method was successfully applied for the determination of vitamin B9 and vitamin B12 in pharmaceutical samples satisfactorily.
KEYWORDS:Vitamin B9 and B12; Simultaneous determination; Multi-wall carbon nanotubes
Download this article as:| Copy the following to cite this article: Shishehbore M. R. Simultaneous Voltammetric Determination of Vitamine B9 and B12 using a Hydroquinone Derivative Multi-Wall Carbon Nanotubes Paste Electrode. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Shishehbore M. R. Simultaneous Voltammetric Determination of Vitamine B9 and B12 using a Hydroquinone Derivative Multi-Wall Carbon Nanotubes Paste Electrode. Orient J Chem 2013;29(2). Available from: http://www.orientjchem.org/?p=22173 |
Introduction
Folic acid, (N-[p-{[(2-amino-4-hydroxy-6-pteridinyl) methyl] amino}benzoyl]-l-glutamic acid, is a water-soluble of vitamin B group (vitamin B9). It is produced by plants (green leaves, algae) and micro-organisms (yeast, bacteria). A lack of vitamin B9 is a common cause of gigantocytic anaemia, and it is thought to increase the likelihood of heart attack and stroke. The Department of Health in UK proposed corroboration of flour with folic acid at 240 mg/100 g 1. Therefore, it is necessary to quantify vitamine B9 with reliable results. In recent years, several methods for the determination of vitamine B9 have been available, including high performance liquid chromatography 2, microemulsion electrokinetic chromatograpy 3 and fluorescence spectroscopy 4. As vitamin B9 is an electro-active compound, some electrochemical methods have been reported for its determination 5-7. Compared to other procedures, electrochemical methods are more desirable and have come to be of growing use in different fields of practice such as environment monitoring, medicine, and biotechnology. This is because of the convenience and low cost of the methods.
Vitamin B12 was discovered from its relationship to the disease pernicious anemia, which is an autoimmune disease in which parietal cells of the stomach responsible for secreting intrinsic factor are destroyed. Intrinsic factor is crucial for the normal absorption of B12, so a lack of intrinsic factor, as seen in pernicious anemia, causes a vitamin B12 deficiency. Many other subtler kinds of vitamin B12 deficiency and their biochemical effects have since been elucidated. Vitamin B12 normally plays a significant role in the metabolism of every cell of the body, especially affecting the DNA synthesis and regulation but also fatty acid synthesis and energy production. However, many (though not all) of the effects of functions of B12 can be replaced by sufficient quantities of vitamin B9 (folic acid). Therefore, simultaneous determination of vitamin B9 and vitamin B12 is very important 8.
Experimental
Chemicals and apparatus
All of the solutions were freshly prepared using double-distilled water. vitamine B9 (Sigma) and vitamin B12 (Merck) with analytical grade used as received. 4-Hydroxy-2-(triphenylphosphonio)phenolate (HTP) was synthesized as reported previously 9. Graphite fine powder (Fluka) and paraffin oil (DC 350, Merck, density = 0.88 g cm−3) were used as binding agents for the graphite pastes. The multi-wall carbon nanotubes (MWCNT) with diameter 10–20 nm, length 5–20 mM and purity of >95% were purchased from Nanolab Inc (Brighton, MA). The buffer solutions were prepared from ortho-phosphoric acid and its salts.
Voltammetric measurements were perfomed using a computerized potentiostat/galvanostat Autolab (model PGSTAT 30, Eco Chemie B.V.A) equipped with General Purpose Electrochemical System (GPES) 4.9 software. A three electrode assembly was employed to the experiment in a 50 mL glass cell containing 4-hydroxy-2-(triphenylphosphonio) phenolate multi-walled carbon nanotubes modified carbon paste electrode (HTP-MWCNT-CPE) as the working electrode, an Ag/AgCl electrode as reference electrode, a graphite counter electrode. All of the potentials were measured and reported versus. Ag/AgCl as reference electrode. A Metrohm 781 pH/ion meter was also used for pH measurements.
Electrode preparation
A 0.5 mg of HTP hand mixed with 2-times its weight of MWCNT and 200-times its weight of graphite powder in a mortar with a pestle. Paraffin was added to the above mixture using a 5 mL syringe and mixed for 20 min until a uniformly wetted paste was obtained. The HTP-MWCNT-CP electrode (HTP-MWCNT-CPE) was fabricated by packing the paste into the end of a Teflon rod (ca. 2mm i.d. and 10 cm long). Then electrical contact was made by inserting a copper wire into the Teflon rod at the end of the mixture. When necessary, a new surface was obtained by pushing an excess of paste out of the tube and polishing it on a weighing paper. Multi-walls carbon nanotubes carbon paste electrode (MWCNT-CPE) and HTP-CPE as modified carbon paste, prepared in the same way without adding HTP and carbon nanotubes to the mixture respectively. Moreover, carbon paste electrode (CPE) was prepared by mixing graphite powder and paraffin to obtain a wetted paste and fabricated as explained.
Pharmaceutical sample preparation
Three vitamin B9 tablets were powdered and mixed thoroughly. An amount corresponding to definite mg of vitamin B9 was weighed, transferred to 1 L volumetric flask and dissolved with a phosphate buffer solution pH 7.0 before the measurements.
The vitamin B12 injection solution was diluted 103 times with a phosphate buffer solution pH 7.0.
Results and Discussion
Electro-catalytic characteristic of vitamin B9 at the HTP-MWCNT-CPE
Cyclic voltammetry was used for studying the efficiency MWCNT and HTP as a modifier. For this purpose, cyclic voltammograms of CPE, MWCNT-CPE, HTP-CPE and HTP-MWCNT-CPE in absence and presence of vitamin B9 were recorded and shown in Fig. 1. A comparison of cyclic voltamograms of Fig. 1 demonstrates the electro-oxidation of vitamie B9 can be catalyzed by a dramatic change in oxidation potential and an increase in the anodic peak current. The electrocatalytic activity discussed in detail as follow:
Curves (a) and (b) of Fig. 1 show the cyclic voltammograms of HTP-MWCNT-CPE in absence (curve a) and presence (curve b) of 0.12 mM of vitamin B9 in 0.15 M phosphate buffer solution pH 7.0 at sweep rate 20 mV s-1. As expected for an electrocatalytic oxidation, there is an increase in peak current at the potential of 220 mV in the presence of vitamine B9 that shown the efficiency of the electrocatalytic oxidation reaction. Under the same experimental conditions, the cyclic voltammograms of HTP-CPE in the absence (curve c) and in the presence (curve d) of 0.12 mM of vitamin B9 are recorded. Considering the oxidation responses of vitamin B9 at HTP-CPE (curve d) and HTP-MWCNT-CPE (curve b), a dramatic enhancement in the anodic peak current at HTP-MWCNT-CPE as compared to the value obtained at HTP-CPE was observed that is due to the increase of surface area of HTP-MWCNT-CPE as compared with HTP-CPE. Similarly, cyclic voltammograms of MWCNT-CPE in 0.12 mM of vitamin B9 (curve f) and in 0.15 M phosphate buffer solution (curve e) were recorded. The cyclic voltammograms of the electrochemical oxidation of 0.1 mM of vitamin B9 at HTP-MWCNT-CPE (curve b) and HTP-CPE (curve d) are appeared at the same potential which is about 220 mV, but there is no anodic peak current at MWCNT-CPE. These results clearly show that combination of MWCNT and HTP definitely improves the sensitivity. Therefore, the electrocatalytic effect is more effective on vitamin B9 using MWCNT and the electrocatalytic effect of HTP-MWCNT-CPE toward vitamin B9 oxidation.
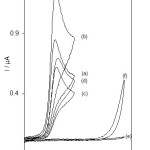 |
Figure 1 |
The cyclic voltammograms of the HTP-MWCNT-CPE at various scan rates recorded in 0.15 M phosphate buffer solution (pH 7.0) containing 0.16 mM of vitamin B9. The obtained cyclic voltammograms shown that the anodic oxidation peak currents of vitamin B9 were proportional linearly to the square root of the scan rate indicating that at sufficiently positive potential the reaction is controlled by the diffusion of vitamin B9. The number of electrons in the overall reaction can be obtained using the slope of IP versus v1/2 plot. Based on the following equation for a totally irreversible diffusion controlled processes:
Ip = 3.01 × 105 n[(1 − α˛)nα]1/2ACbv1/2D1/2 (1)
and considering (1 − α)nα = 0.63 (as mentioned below), D = 1.76 × 10-5 (that was obtained by chronoamperometry) and A = 0.0314 cm2, the total number of electrons of the anodic oxidation of vitamin B9 is n= 1.65 @ 2.
Chronoamperometric studies
Chronoamperometry as an effective electrochemical technique was employed for the investigation of electrode processes at chemically modified electrodes. Fig. 2 shows current-time profiles obtained by setting the working electrode potential at 225 mV for different concentrations of vitamin B9. For vitamin B9 as an electroactive material with a diffusion coefficient of D, the current for the electrochemical reaction is described by the Cottrell equation:
I = nFAD1/2Cbπ-1/2t-1/2 (2)
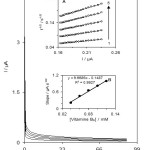 |
Figure 2 |
where D and Cb are the diffusion coefficient (cm2 s−1) and the bulk concentration (molcm−3) respectively. At long experimental times (t > 0.1 s), where the electro-catalyzed oxidation rate of vitamin B9 exceeds that of vitamin B9 diffusion, the current has a diffusional nature From the slopes, the mean value of D was found to be 1.76 × 10−5 cm2 s-1 for vitamin B9.
DPV technique for quantification of vitamin B9 and vitamin B12
The main objective of this study was the development of a modified electrode capable of electrocatalytic oxidation of vitamin B9 and simultaneous determination of vitamin B9 and vitamin B12. Differential pulse voltammetry (DPV), which has a much higher current sensitivity than cyclic voltammetry and lower charging current contribution to the background current, was used to estimate the linear range of vitamin B9 and lower limit of detection. The effects of increasing the vitamin B9 concentration on the voltammograms are investigated. Linear calibration curve was obtained over the range 1.5-135.3 µM. The lower limit of detection was obtained according to the equation Cm =3sb/m where sb is the standard deviation of the blank response (µA) and m is the slope of the calibration plot. The data analysis presents the value of lower limit detection of vitamin B9 to be 0.2 µM.
The utilization of the modified electrode was investigated for the simultaneous determination of vitamin B9 and vitamin B12. For this purpose, the concentrations of vitamin B9 and vitamin B12 were changed simultaneously. The voltammetric response show that the simultaneous determination of vitamin B9 and vitamin B12 with two anodic peaks at potentials of 65 and 145 mV, corresponding to the oxidation of vitamin B9 and vitamin B12, is possible at HTP-MWCNT-CPE. The plot of peak current versus vitamin B9 concentration is linear in the range 5.3-62.5 µM. Also, the linear correlation between peak current and vitamin B12 concentration in the range 6.2-75.0 µM with the same sensitivities.
Application of the modified electrode for determination of vitamin B9 and vitamin B12 in pharmaceutical samples
Applicability and reliability of the proposed modified electrode in real samples with different matrixes was tested to confirm the usefulness of HTP-MWCNT-CPE. Vitamin B9 and vitamin B12 oral injection was used as pharmaceutical sample. Sample preparation was done as previously discussed and DPVs were recorded for estimating the vitamin B9 and vitamin B12 concentration using calibration curves. The results were shown in Table 1. As it can be seen, the total value of vitamin B9 and vitamin B12 that was found is in agreement with that registered in the label of the pharmaceutical inhalation product. Since the obtained results are in a good agreement with those declared on the label of the pharmaceutical inhalation products, the reliability of the proposed modified electrode was confirmed.
Table 1: Determination of vitamin B9 and vitamin B12 in pharmaceutical samples.
|
Sample |
Added / µM |
Founda / µM |
Recovery (%) |
RSD (%) |
Total valueb |
Declared value |
||||||
|
V B9 |
V B12 |
V B9 |
V B12 |
V B9 |
V B12 |
V B9 |
V B12 |
V B9 (mg) |
V B12 (mg mL-1) |
V B9 (mg) |
V B12 (mg mL-1) |
|
|
Vitamin B9 tablet |
– |
– |
5.19 |
– |
– |
– |
2.3 |
– |
4.96 |
0.98 |
5.0 |
1.0 |
|
5.0 |
– |
10.11 |
– |
99.2 |
– |
1.9 |
– |
– |
– |
– |
– |
|
|
Vitamin B12 injection solution |
– |
– |
– |
0.96 |
– |
– |
– |
2.2 |
– |
– |
– |
– |
|
– |
5.0 |
– |
5.88 |
– |
98.6 |
– |
2.1 |
– |
– |
– |
– |
|
a Average of three replicate measurement.
b The total values were calculated by multiplying the measured values by the appropriate dilution factor (n=3).
Conclusions
This study demonstrates the application of HTP-MWCNT-CPE in individually and simultaneous determination of vitamin B9 and vitamin B12. The modified electrode exhibited good electrocatalytic activity toward the electro-oxidation of vitamin B9 at pH 7.0. The transfer coefficient, α, and the overall number of electrons involved in the electrocatalytic oxidation of vitamin B9 at the modified electrode surface were determined using voltammetric methods. Chronoamperometry was used for the determination of diffusion coefficient. Using differential pulse voltammetry, linear calibration ranges were obtained for vitamin B9 and vitamin B12. The proposed modified electrode was successfully applied for the determination of vitamin B9 and vitamin B12 in urine and pharmaceutical samples.
Acknowledgments
The authors are thankful to the research council of the Islamic Azad University (Yazd branch) for financial supporting of this research.
References
- A.J.A. Wright, P.M. Finglas, S. Southon, Trends in Food Science & Technology 12(9): 313-321 (2001).
- A.R. Bernaldo de Quiros, C. Castro de Ron, J. Lopez-Hernandez, M.A. Lage-Yusty, J. Chromatogr. A, 1032(1-2): 135-139 (2004).
- M.S.A. Pradoa, C.A. Silva, M.F.M. Tavaresa, K.D. Altriab, J. Chromatogr. A, 1051(1-2): 291-296 (2004):
- J.C. Huang, D. Li, J. Diao, J. Hou, J.L. Yuan, G.L. Zou, Talanta, 72(4): 1283-1287 (2007).
- B.B. Prasad, M.P. Tiwari, R. Madhuri, P.S. Sharma, Anal. Chim. Acta 662(1): 14-22 (2010).
- J.M. Fernandez, A.C. Garcia, A.J.M Oridieres, P.T.J. Blanco, J. Electroanal. Chem. 225(1-2): 241-253 (1987).
- T.J. Oshea, A.C. Garcia, P.T. Blanco, J. ElectroanaL. Chem., 307(1-2): 63-71 (1991).
- P. Berton, R.P. Monasterio, R.G. Wuilloud, Talanta 97(1): 521-526 (2012).
- D. Nematollahi, E. Tammari, R. Esmaili, J. Electroanal. Chem. 621(1): 113-116 (2008).

This work is licensed under a Creative Commons Attribution 4.0 International License.









