Reduction of Carbonyl Compounds to their Corresponding of Alcohols with [Zn(BH4)2(2-MeOpy)] & [Zn(BH4)2(2-Mepy)] as New Reducing Agents (A Comparison Study)
Behrooz Khezri , Farnaz Najaf Ghadimi , Chonur Nevisandeh Karashi and Davood Setamdideh*
Department of Chemistry, Faculty of Sciences, Mahabad Branch, Islamic Azad University, Mahabad - 59135-443, Iran.
Corresponding Author E-mail: davood.setamdideh@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/290233
The reduction of a variety of carbonyl compounds was efficiently carried out with [Zn(BH4)2(2-MeOpy)] and [Zn(BH4)2(2-Mepy)] as new reducing agents. The reduction reactions were performed to give the corresponding alcohols derivatives in perfect yields.
KEYWORDS:Zinc borohydride; Reduction; Carbonyl compounds; Chemoselective; Regioselectivity
Download this article as:| Copy the following to cite this article: Khezri B, Ghadimi F. N, Karashi C. N, Setamdideh D. Reduction of Carbonyl Compounds to their Corresponding of Alcohols with [Zn(BH4)2(2-MeOpy)] & [Zn(BH4)2(2-Mepy)] as New Reducing Agents (A Comparison Study). Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Khezri B, Ghadimi F. N, Karashi C. N, Setamdideh D. Reduction of Carbonyl Compounds to their Corresponding of Alcohols with [Zn(BH4)2(2-MeOpy)] & [Zn(BH4)2(2-Mepy)] as New Reducing Agents (A Comparison Study). Orient J Chem 2013;29(2). Available from: http://www.orientjchem.org/?p=22193 |
Introduction
Ranu et al has reported Zn(BH4)2 capable of reducing some carbonyl compounds 1a-c.However, zinc tetrahydroborate has been used less than regular reducing agents in laboratory for the reduction of organic compounds, because of non-availability as a commercial reagent, being freshly prepared solution just prior to use and limitation to handling and storage 2. Therefore to overcome this problems some modifications of zinc tetrahydroborate such as [Zn(BH4)2(dabco)] 3, [Zn(BH4)2(pyz)]n4, [Zn(BH4)2(Ph3P)] &[Zn(BH4)2(Ph3P)2] 5, [Zn(BH4)2(bpy)] 6, [Zn(BH4)2(py)] 7, [Zn(BH4)2XP4] 8, [Zn(BH4)2(nmi)] 9 and [Zn(BH4)2(nic)] 2 have been made and used. In continuation of our interest for preparation of new zinc tetrahydroborate systems 10, we now wish to report the preparation of new stable ligand-zinc tetrahydroborates i.e (2-methoxypiridine) (tetrahydroborato)zinc complex, [Zn(BH4)2(2-MeOpy)] and (2-methylypiridine) (tetrahydroborato)zinc complex, [Zn(BH4)2(2-Mepy)] and their reducing ability in the reduction of carbonyl compounds such as aldehydes, ketones and acyloins, a-diketones to their corresponding alcohols.
Experimental
General
All substrates and reagents were purchased from commercially sources with the best quality and used without further purification. IR and 1H NMR spectra were recorded on PerkinElmer FT-IR RXI and 400 MHz Bruker spectrometers, respectively. The products were characterized by their 1H NMR or IR spectra and comparison with authentic samples (melting or boiling points). Organic layers were dried over anhydrous sodium sulfate. All yields referred to isolated pure products. TLC was applied for the purity determination of substrates, products and reaction monitoring over silica gel 60 F254 aluminum sheet.
(2-methoxypiridine)(tetrahydroborato)zinc complex; [Zn(BH4)2(2-MeOpy)]
An ethereal solution of Zn(BH4)2 (0.16 M, 250 mL) was prepared from ZnCl2(5.452 g, 0.04 mol) and NaBH4 (3.177 g, 0.084 mol) according to an available procedure in the literature. 10 Then, 2-methoxypiridine (4.36 g, 0.04 mol) in ether (50 mL) was added dropwise to the ethereal solution of Zn(BH4)2 and stirred for 30 min. Evaporation of the solvent under vacuum at room temperature gave [Zn(BH4)2(2-MeOpy)] as a orange powder in a quantitative yield (8.00 g, 98%). Found: Zn: 31.71 %, B: 10.06 %. Calculated for C6H15B2NOZn, Zn: 39.02 %, B: 10.59%.
(2-methoxypiridine)(tetrahydroborato)zinc complex; [Zn(BH4)2(2-Mepy)]
An ethereal solution of Zn(BH4)2 (0.8 M, 150 mL) was prepared from ZnCl2(2.73 g, 0.02 mol) and NaBH4 (1.59 g, 0.042 mol) according to an available procedure in the literature [10]. Then, 2-methylpiridine (1.86 g, 0.02 mol) in ether (30 mL) was added dropwise to the ethereal solution of Zn(BH4)2 and stirred for 30 min. Evaporation of the solvent under vacuum at room temperature gave [Zn(BH4)2(2-Mepy)] as a creamy gel a quantitative yield (3.57 g, 95%). Found: Zn: 34.40 %, B: 11.06 %. Calculated for C6H15B2NOZn, Zn: 34.74 %, B: 11.49%.
Reduction of Benzaldehyde to Benzyl alcohol with [Zn(BH4)2(2-MeOpy)], A Typical Procedure
In a round-bottomed flask (10 mL), equipped with a magnetic stirrer, a solution of banzaldehye (0.106 g, l mmol) in CH3CN (3 mL) was prepared. The complex reducing agent (0.1 g, 0.5 mmol) was then added and the mixture was stirred at room temperature. TLC monitored the progress of the reaction (eluent; Hexane/EtOAc: 9/1). After completion of the reaction within 1 min, a solution of 5% HCl (5 mL) was added to the reaction mixture and stirred for 5 min. The mixture was extracted with CH2Cl2 (3 × 10 mL) and dried over the anhydrous sodium sulfate. Evaporation of the solvent and short column chromatography of the resulting crude material over silica gel (0.015-0.040 mm) by eluent of (Hexane/EtOAc: 9/1) afforded the pure liquid benzyl alcohol (0.105 g, 98% yield)
Reduction of Benzaldehyde to Benzyl alcohol with [Zn(BH4)2(2-Mepy)], A Typical Procedure
In a round-bottomed flask (10 mL), equipped with a magnetic stirrer, a solution of banzaldehye (0.106 g, l mmol) in CH3CN (3 mL) was prepared. The complex [Zn(BH4)2(2-Mepy)] agent (0.095 g, 0.5 mmol) was then added and the mixture was stirred at room temperature. TLC monitored the progress of the reaction (eluent; Hexane/EtOAc: 9/1). After completion of the reaction less than 2 min, a solution of 5% HCl (5 mL) was added to the reaction mixture and stirred for 5 min. The mixture was extracted with CH2Cl2 (3 × 10 mL) and dried over the anhydrous sodium sulfate. Evaporation of the solvent and short column chromatography of the resulting crude material over silica gel (0.015-0.040 mm) by eluent of (Hexane/EtOAc: 9/1) afforded the pure liquid benzyl alcohol (0.102 g, 95% yield).
Results and Discusion
(Pyridine)(tetrahydroborato)zinc complex [Zn(BH4)2(py)] (Scheme 1, A) has used for the some reduction purpose such as reduction of organic carbonyl compounds to their corresponding alcohols, reduction of carboxylic acids to their corresponding alcohols and reduction of nitro compounds to their corresponding amines 7. Also we have used it for the reductive acetylation of organic carbonyl compounds 11. On the other hand, (nicotine)(tetrahydroborato)zinc complex [Zn(BH4)2(nic)] (Scheme 2, B) has been synthesis as chiral reducing agent 2. This reducing agent has used for the reduction of carbonyl compounds to their corresponding alcohols. For the investigation of effect of electron-donating groups on reducing power of [Zn(BH4)2(py)], we have prepared [Zn(BH4)2(2-MeOpy)] and [Zn(BH4)2(2-Mepy)] as new stable orange solid and cream gel respectively. They can be readily prepared by complexation of 1: 1 ethereal solution of zinc tetrahydroborate and 2-methoxypiridine or 2-methylpiridine at room temperature. The complexes are readily formed quantitatively. Filtration and evaporation of the solvent result in stable complexes which could be stored in a sealed bottle for months without losing their activity. The Zn content in the complexes is determined by both gravimetric and atomic absorption techniques. The measurements data are in good agreement with the proposed structure of the reagent as [Zn(BH4)2(2-MeOpy)] (Scheme 3, C) and [Zn(BH4)2(2-Mepy)] (Scheme 4, D).
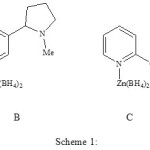 |
Scheme 1 Click here to View scheme |
[Zn(BH4)2(2-MeOpy)] and [Zn(BH4)2(2-Mepy)] in protic solvents such as water, ethanol and methanol are decomposed with the evolution of hydrogen gas. Also, these reagents are slightly soluble in various aprotic solvents such as Et2O, CH2Cl2, CHCl3, CH3CN and THF. Reduction of aldehydes and ketones to their corresponding alcohols could be easily achieved by [Zn(BH4)2(2-MeOpy)] and [Zn(BH4)2(2-Mepy)]. For the selection of appropriate conditions in reduction reactions, benzaldehyde and acetophenone as model compounds were adopted in different solvents. Our observation reveals that the reduction of carbonyl compounds in CH3CN provided a fast reaction rate and efficiency as shown in scheme 2 and scheme 3, (Table 1, entries 2 and 7).
Table 1: Optimaization of the Reduction Reactions of Benzaldehyde to Benzyl alcohol and Acetophenone to 1-Phenylethanol with [Zn(BH4)2(2-MeOpy)] and [Zn(BH4)2(2-Mepy)] as Reducing Agents at Room Temperature.
|
Entry |
Substrate |
Molar Ratio Substrates/Reagents |
Solvent |
[Zn(BH4)2(2-MeOpy)] |
[Zn(BH4)2(2-Mepy)] |
||
|
Time (min) |
Conversiona/% |
Time/min |
Conversiona/% |
||||
|
1 |
benzaldehyde |
1:1 |
CH3CN |
1< |
100 |
1< |
100 |
|
2 |
benzaldehyde |
1:0.5 |
CH3CN |
1< |
100 |
2 |
100 |
|
3 |
benzaldehyde |
1:0.25 |
CH3CN |
60 |
100< |
60 |
100< |
|
4 |
benzaldehyde |
1:0.5 |
CH2Cl2 |
60 |
100< |
60 |
100< |
|
5 |
benzaldehyde |
1:0.5 |
Et2O |
30 |
100 |
45 |
100 |
|
6 |
benzaldehyde |
1:0.5 |
THF |
15 |
100 |
5 |
100 |
|
7 |
acetophenone |
1:1 |
CH3CN |
10 |
100 |
18 |
100 |
|
8 |
acetophenone |
1:1 |
THF |
30 |
100 |
50 |
100 |
|
9 |
acetophenone |
1:1 |
Et2O |
60 |
100< |
60 |
100< |
|
10 |
acetophenone |
1:0.5 |
CH3CN |
60 |
100< |
60 |
100< |
a Completion of the reactions were monitored by TLC (eluent; Hexane/EtOAc: 9/1).
 |
Scheme 2:
|
 |
Scheme 3 Click here to View scheme |
Reduction of a variety of structurally different aromatic and aliphatic aldehydes to their corresponding alcohols is performed efficiently with this reducing agent (Table 2). Aldehydes are reduced with 0.5 molar amounts of the reagents in CH3CN at room temperature in high to excellent yields (90-98%). Reduction of ketones is also performed well with 1 molar amounts of the reagents at room temperature in CH3CN. The efficiency of these reactions were also excellent (90-98%) (Table 3). On the other hands, reduction of α-diketones usually gives a mixture of α-hydroxy ketones and vicinal diols. Reduction of α-diketones is easily achieved by [Zn(BH4)2(2-MeOpy)] and [Zn(BH4)2(2-Mepy)]. These reagents with 1 molar equivalents efficiently reduces α-diketones to their vicinal diols in CH3CN at room temperature (Table 4, entries 1, 3 and 5). Our attempts for reduction of α-diketones to acyloins were unsatisfactory and only vicinal diols were detected as products. In addition to the reduction of acyloins to vicinal diols is also important in organic synthesis. In continuation of our study, this goal also easily achieved by [Zn(BH4)2(2-MeOpy)] and [Zn(BH4)2(2-Mepy)] efficiently at temperature in CH3CN by utilizing 1 molar equivalents of the reagents (Table 4, entries 2 and 4). Regioselective 1,2-reduction of α,β-unsaturated aldehydes and ketones to give allylic alcohols is a widely used transformation in organic synthesis which is achieved with various hydride transferring agents.The regioselectivity of the [Zn(BH4)2(2-MeOpy)] [Zn(BH4)2(2-Mepy)] reagents was examined with the reduction of cinnamaldehyde as a model compound in CH3CN. The reduction was carried out with the 0.5 molar ratio of [Zn(BH4)2(2-MeOpy)] and [Zn(BH4)2(2-Mepy)] in CH3CN at room temperature (Table 4, entry 6). The result shows that the product cinnamyl alcohol was obtained perfectly by these reducing agents. These reagents were also applied for the reduction of citral at room temperature (Table 4, entry 7). Reduction of conjugated enones took place with 1 molar equivalents of [Zn(BH4)2(2-MeOpy)] and [Zn(BH4)2(2-Mepy)] in CH3CN at room temperature. Benzalacetone, benzalacetophenone and β-ionone were the examples which were reduced by [Zn(BH4)2(2-MeOpy)] and [Zn(BH4)2(2-Mepy)] reagents with perfect regioselectivity and efficiency in CH3CN (Table 4, entries 8-10). The corresponding allyl alcohols were obtained regioselectively in perfect yields. In order to show the efficiency of the reagents, we compared our results with [Zn(BH4)2(py)] and [Zn(BH4)2(nic)] as shown in Table 5. In the all cases [Zn(BH4)2(2-MeOpy)] and [Zn(BH4)2(2-Mepy)] show better results.
Table 2: Reduction of Aldehydes (1 mmol) with [Zn(BH4)2(2-MeOpy)] (0.5 mmol) and [Zn(BH4)2(2-Mepy)] (0.5 mmol) in CH3CN at Room Temperature.
|
Entry |
Substrate |
Product |
[Zn(BH4)2(2-MeOpy)] |
[Zn(BH4)2(2-Mepy)] |
||
|
Time/min |
Yield/%a |
Time/min |
Yield/%a |
|||
|
1 |
benzaldehyde |
benzyl alcohol |
Imb |
98 |
2 |
95 |
|
2 |
2-bromobenzaldehyde |
2-bromobenzyl alcohol |
Im |
96 |
2 |
92 |
|
3 |
4-bromobenzaldehyde |
4-bromobenzyl alcohol |
Im |
90 |
2 |
94 |
|
4 |
4-chlorobenzaldehyde |
4-clorobenzyl alcohol |
Im |
96 |
2 |
96 |
|
5 |
4-methylbenzaldehyde |
4-methylbenzyl alcohol |
Im |
94 |
5 |
91 |
|
6 |
4-methoxybenzaldehyde |
4-methoxybenzyl alcohol |
1 |
93 |
2 |
93 |
|
7 |
2-methoxybenzaldehyde |
2-methoxybenzyl alcohol |
1 |
97 |
5 |
93 |
|
8 |
3-methylbenzaldehyde |
3-methylbenzyl alcohol |
Im |
95 |
5 |
92 |
|
9 |
2-methylbenzaldehyde |
2-methylbenzyl alcohol |
Im |
97 |
5 |
92 |
|
10 |
4-nitrobenzaldehyde |
4-nitrobenzyl alcohol |
Im |
95 |
2 |
94 |
|
11 |
2-nitrobenzaldehyde |
2-nitrobenzyl alcohol |
Im |
98 |
2 |
97 |
|
12 |
1-butanal |
1-butanol |
Im |
96 |
2 |
95 |
aYields refer to isolated pure products.b Im: immediately.
Table 3: Reduction of Ketones (1 mmol) with [Zn(BH4)2(2-MeOpy)] (1 mmol) and [Zn(BH4)2(2-Mepy)] (1 mmol) in CH3CN at Room Temperature.
|
Entry |
Substrate |
Product |
[Zn(BH4)2(2-MeOpy)] |
[Zn(BH4)2(2-Mepy)] |
||
|
Time/min |
Yield/%a |
Time/min |
Yield/%a |
|||
|
1 |
benzophenone |
diphenylmethanol |
80 |
98 |
90 |
92 |
|
2 |
acetophenone |
1-phenylethanol |
10 |
93 |
18 |
98 |
|
3 |
4-bromoacetophenone |
1-(4–bromophenyl)ethanol |
10 |
96 |
15 |
94 |
|
4 |
4-methoxyacetophenone |
1–(4–ethoxyaphenyl)ethanol |
90 |
97 |
100 |
96 |
|
5 |
4-methylacetophenone |
1–(4–methylphenyl)ethanol |
30 |
95 |
40 |
94 |
|
6 |
4-methoxybenzophenone |
(4-methoxyphenyl) (phenyl) methanol |
120 |
96 |
180 |
90 |
|
7 |
4-nitroacetophenone |
1–(4–notrophenyl)ethanol |
10 |
93 |
15 |
93 |
|
8 |
4-chloroacetophenone |
1–(4–chlorophenyl)ethanol |
10 |
94 |
15 |
98 |
|
9 |
2,3-dihyroinden-1-one |
2,3-dihydro-1H-inden-1-ol |
80 |
96 |
90 |
93 |
|
10 |
9H-fluoren-9-one |
9H-fluoren-9-ol |
180 |
93 |
210 |
95 |
|
11 |
2-methylcyclohexanone |
2-methylcyclohexanol |
5 |
94 |
5 |
97 |
|
12 |
cyclohexanone |
cyclohexanol |
5 |
98 |
5 |
98 |
|
13 |
4-phenylcyclohexanone |
4-phenylcyclohexanol |
5 |
98 |
5 |
92 |
|
14 |
3-pentanone |
3-pentanol |
5 |
97 |
5 |
94 |
|
15 |
4-phenyl-2-butanone |
4-phenylbutan-2-ol |
5 |
96 |
5 |
91 |
aYields refer to isolated pure products.b Im: immediately.
Table 4: Reduction of Acyloines (1 mmol), α-Dicarbonyles (1 mmol), Enals (1 mmol), and Enones (1 mmol) with [Zn(BH4)2(2-MeOpy)] (1 mmol) and [Zn(BH4)2(2-Mepy)] (1 mmol) in CH3CN at RoomTemperature.
|
Entry |
Substrate |
Product |
[Zn(BH4)2(2-MeOpy)] |
[Zn(BH4)2(2-Mepy)] |
||
|
Time/min |
Yield/%a |
Time/min |
Yield/%a |
|||
|
1 |
benzil |
1,2-diphenyl ethane-1,2-diol |
5 |
98 |
15 |
95 |
|
2 |
benzoin |
1,2-diphenyl ethane-1,2-diol |
5 |
95 |
25 |
94 |
|
3 |
1,2-bis(4-methoxyphenyl)ethane-1,2- dione |
1,2-bis(4-methoxyphenyl)ethane-1,2-diol |
8 |
97 |
20 |
97 |
|
4 |
2-hydroxy-1,2-bis(4-methoxyphenyl) ethanone |
1,2-bis(4-methoxyphenyl)ethane-1,2-diol |
8 |
94 |
40 |
94 |
|
5 |
1,3-diphenylpropane-1,2-dione |
1,3-diphenylpropane-1,2-diol |
10 |
96 |
50 |
97 |
|
6b |
cinnamaldehyde |
3-phenyl-2-propen-1-ol |
1 |
96 |
2 |
96 |
|
7b |
citral |
3,7-dimethyl-2,6-octadien-1-ol |
1 |
93 |
2 |
93 |
|
8 |
benzylideneacetone |
4-phenyl-3-buten-2-ol |
15 |
98 |
25 |
98 |
|
9 |
chalcone |
1,3-dipheny-2-propen-1-ol |
70 |
96 |
90 |
93 |
|
10 |
β-ionone |
4-(2,6,6-trimethylcyclohex-1-enyl)-3-buten-2-ol |
15 |
97 |
20 |
97 |
aYields refer to isolated pure products.b The Reduction reaction was carried out with 0.5 molar equivalents of [Zn(BH4)2(2-MeOpy)] and [Zn(BH4)2(2-Mepy)].
Table 5: Comparisons of the Reduction of Carbonyl Compounds with [Zn(BH4)2(2-MeOpy)], [Zn(BH4)2(2-Mepy)], [Zn(BH4)2(py)] and [Zn(BH4)2(nic)]
|
Substrate |
Molar Ratio (Reagent./Substrate), Time (h) |
||||
|
[Zn(BH4)2(2-MeOpy)] |
[Zn(BH4)2(2-Mepy)] |
[Zn(BH4)2(py)] |
[Zn(BH4)2(nic)] |
||
|
benzaldehyde |
0.5:1, Ima |
0.5:1, 0.03a |
1:1, 0.5a |
1:1, 0.08a |
|
|
4-methoxybenzaldehyde |
0.5:1, 0.02a |
0.5:1, 0.08a |
1:1, 1.3a |
1:1, 0.41a |
|
|
acetophenone |
1:1, 0.16a |
1:1, 0.3a |
– |
2:1, 1.3a |
|
|
benzophenone |
1:1, 1.3a |
1:1, 1.5a |
2:1, 4.3b |
2:1, 2.75a |
|
|
cyclohexanone |
1:1, 0.08a |
1:1, 0.08a |
2:1, 2b |
2:1, 0.16a |
|
|
9H-fluoren-9-one |
1:1, 3a |
1:1, 3.5a |
2:1, 5.3b |
2:1, 4.5a |
|
a The reduction reactions have been carried out at room temperature. b The reduction reactions have been carried out under reflux conditions. cIm: immediately.
Conclusion
In conclusion we have shown that the reduction of a variety of carbonyl compounds was efficiently carried out with [Zn(BH4)2(2-MeOpy)] and [Zn(BH4)2(2-Mepy)] as new reducing agents. The reactions were performed to give the corresponding alcohols in perfect yields in CH3CN at room temperature. Reduction of acyloins and α-diketones by these reducing agents produced efficiently the corresponding vicinal diols. Regioselectivity of these reagents was also investigated with exclusive 1,2-reduction of conjugated carbonyl compounds to their corresponding allylic alcohols in high to excellent yields. High efficiency of the reduction reactions, shorter reaction times, using less equivalents of the reagents for the reduction reactions, mild conditions and easy work-up procedure makes as attractive new reducing agents for reduction of carbonyl compounds and a synthetically useful metal tetrahydroborate complexes.
Acknowledgments
The authors gratefully appreciated the financial support of this, work by the research council of Islamic Azad University branch of Mahabad.
Refrences
- Ranu, B. C. Synlett. 885 (1993). b) Ranu, B. C.; Chakraborty, R. , Tetrahedron Lett. 31: 7663 (1990). c) Sarkar, D. C.; Das, A. R.; Ranu, B. C. J. Org. Chem. 55: 5779 (1990).
- Setamdideh, D.; Rafigh, M. E-J. Chem, 4: 2338 (2012).
- Firouzabadi, H.; Adibi, M.; Zeynizadeh,B. Synth. Commun. 28: 1257 (1998).
- Tamami, B.; Lakouraj, M. M. Synth. Commun. 25: 3089 (1995).
- Firouzabadi, H.; Adibi, M.; Ghadami, M. Phosphorus, Sulfur, Silicon Rel. Elem. 142: 191 (1998).
- Zeynizadeh, B. Bull. Chem. Soc. Jpn. 76: 317 (2003).
- ZeynizadehB.; Faraji, F. Bull. Korean Chem. Soc. 24: 453 (2003). (b) Zeynizadeh, B.; Zahmatkesh, K. J. Chin. Chem. Soc. 50: 267 (2003). (c) Zeynizadeh, B.; Zahmatkesh, K. J. Chin. Chem. Soc. 51: 801(2004). (d) Zeynizadeh, B.; Zahmatkesh, K. J. Chin. Chem. Soc. 52:109 (2005).
- Firouzabadi, H.; TamamiB.; Goudarzian,N. Synth. Commun. 21: 2275 (1991).
- Zeynizadeh, B.; Setamdideh, D. Asian J. Chem. 21: 3603 (2009).
- Setamdideh, D.; Khezri, B.; Rahmatollahzadeh, M.; Aliporamjad, A. Asian J. Chem. 24: 3591 (2012). b) Setamdideh, D.; Rahmatollahzadeh, M. J. Mex. Chem. Soc. 56: 169 (2012). c) Setamdideh, D.; Khezri, B.; Rahmatollahzadeh, M. J. Serb. Chem. Soc. 78: 1 (2013). e) Setamdideh, D.; Karimi, Z.; Rahimi, F. Orient. J. Chem. 27: 1621 (2011).
- Zeynizadeh, B.; Setamdideh, D.; Faraji, F. Bull. Korean Chem. Soc., 29: 76 (2007).

This work is licensed under a Creative Commons Attribution 4.0 International License.









