Phytochemical Analysis of Cold Toluene Extracts of Piper nigrum and its Antibacterial and Antifungal Activity
Monika Gupta*, Aditi Gupta, Sudhakar Gupta, Shweta Thakur and Anuradha Sharma
Department of Chemistry, Lovely Professional University, Phagwara - 144 402, India.
Corresponding Author E-mail: dr.guptmonika@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/290224
A crystalline compound [{(1,5-(1,3)benzodioxol-5yl)-1-oxo-2,4pentadienyl}-piperidine] and few sesquiterpenes ( Caryophyllene, β–bisabolene, δ –Cadinene, Elemol, (-)- Cedreanol, α- Eudesmol ) were obtained from the toluene extract of dried fruits of Piper nigrum. This is for the first time piperine has been extracted from the cold toluene extract of Piper nigrum along with few other sesquiterpenes. Piperine is an alkaloid found naturally in plants belonging to the pyridine group of piperaceae family, mainly responsible for the pungent taste of Piper nigrum where as the components isolated from its oil are meant for aroma. Structure of these compounds is examined and concluded by UV, IR, NMR and MS spectral analysis. The oil separated from toluene extract was analyzed by GC-MS spectroscopy. The components identified by GC-MS spectra, show that caryophyllene was present as major component and δ–Cadinene as minor component. The present study was aimed to extract the phytochemical compounds in toluene extract of Piper nigrum and to check the antibacterial and antifungal activity of the extracts.
KEYWORDS:Piper nigrum; dried fruits; GC-MS; amide; antibacterial activity and antifungal activity
Download this article as:| Copy the following to cite this article: Gupta M, Gupta A, Gupta S, Thakur S, Sharma A. Phytochemical Analysis of Cold Toluene Extracts of Piper nigrum and its Antibacterial and Antifungal Activity. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Gupta M, Gupta A, Gupta S, Thakur S, Sharma A. Phytochemical Analysis of Cold Toluene Extracts of Piper nigrum and its Antibacterial and Antifungal Activity. Orient J Chem 2013;29(2). Available from: http://www.orientjchem.org/?p=22161 |
Introduction
Among many plants of family piperaceae, one showing tremendous potential in therapeutics is Piper nigrum[1]. Piper has 700 species distributed in both hemispheres [2]. As the plant has great adaptability towards various soils and climatic conditions so there will be a good inter species diversity [3]. Piper nigrum also known as black pepper is a climbing perennial shrub, can be used as a spice and folk medicine [2]. Piperine, a piperidine derivative has many pharmacological activities like analgesic, antipyretic, CNS depressant, anti inflammatory, antioxidant, anticonvulsant, antibacterial, antitumor and hepatoprotective [2]. Piper nigrum originated in India [5]. Piperamides are the compounds with greatest insecticidal activities [6]. It has a great commercial, economical and medicinal value [5].
Materials and Methods
Seeds of Piper nigrum is purchased from the authorized seed shop at Jammu and identified by Dr. Gurdev Singh of botany department of Lovely professional University, Phagwara.
Extraction and Isolation
During the research for novel, bio active natural products, the dried and crushed seeds of the plant were successively soxhlated with the light petroleum ether, toluene, chloroform and alcohol on their polarity gradient. All the extracts were showing the potential for further treatment. Toluene extract kept for around three and half months, separated into upper brown coloured oily layer and rod shaped shiny crystals settled at bottom of the beaker. Shiny, pale yellow and rod shaped crystals settled at bottom of the beaker which were further identified on the basis of their spectral studies.
Results and discussion
The shiny pale yellow crystals (m. p. 132°C) obtained from toluene extract of Piper nigrum fruits is found to be an alkaloid on performing Mayer’s reagent test [7]. The molecular formula was established as C17H19O3N by Agilent, 6540 Q-TOF (HRMS) mass spectrometer [8] and the TLC of the pure crystals showed Rf value 0.23 which is very close to standard Rf value of piperine, reported in literature that is 0.25 [4], thus crystals obtained may be of piperine. A UV spectrum shows λmax at 314.5 suggestive of alkaloid framework. IR spectra is compared with the original spectra of piperine [9] that showed absorption bands at 3000 cm-1 corresponds to aromatic C-H stretch; at 1633 and 1610 cm-1 corresponds to symmetric and asymmetric stretching of C=C (diene); 1610,1583 and 1491 cm-1 corresponds to aromatic stretching of C=C phenyl ring ; 1633 cm-1 corresponds to stretching of -CO-N , 2939, 2856 cm-1 corresponds to CH2 asymmetric and symmetric stretching, 1446 cm-1 corresponds to CH2 bending; 1251,1193 cm-1 corresponds to asymmetric stretching of =C-O-C; 1030 cm-1corresponds to symmetric stretching of =C-O-C; 927 cm-1 corresponds to C-O stretching, 1132 cm-1 corresponds to in plane bending of phenyl CH ; 997cm-1 corresponds to C-H bending for trans –CH=CH- ;848, 830 and 804 cm-1 corresponds to out of plane C-H bending. The 400 MHz 1H-NMR spectra showed the peaks at δ 5.976(2H, 7’), δ 7.39 (1H, 3), δ 6.43 (1H, 2), δ 3.529-3.633 (4H,C) and δ 1.562-1.688 (5H, b, a). The 13C-NMR spectra showed the peaks at δ 167.7 (C-1), δ 120.6 (C-2), δ 140.2 (C-3), δ 123.9 (C-4),δ 132.4 (C-1’), δ 106.7 (C-2’), δ 149.7- 149.8 (C-3’,C-4’), δ 109.4 (C-5’), δ 207 (C-6’) and δ 102.7 (C-7’). The HR-mass spectrum showed a molecular ion peak at [M+H]+ 286.14, [2M+H]+ peak at 571.27 and [2M+Na]+ peak at 593.25.
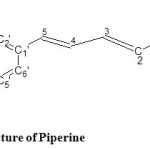 |
Figure 1: Structure of Piperine. Click here to View figure |
Piperine
Pale yellow, rod shaped crystals of toluene extract, exhibited melting point 132℃,UV λmax at 314.5,IR νmax cm1 :3000, 2939, 2856, 1633, 1610, 1583, 1491, 1446 , 1251 ,1193, 1132, 1030, 997 ,927, 830, 804. The 1H-NMR spectra showed the peaks at δ 5.976, δ7.39, δ 6.43, δ 3.529-3.633, δ 1.562-1.688. The 13C-NMR spectra showed the peaks at δ 167.7, δ 120. 6, δ 140.2, δ 123.9, δ 132.4, δ 106.7, δ 149.7-149.8, δ 109.4 , δ 207, δ 102.7. The mass spectrum showed a molecular ion [M+H] + peak at 286.14, [2M + H]+ peak at 571.27 and [2M + Na]+ peak at 593.25.
Analysis of oily fraction
Compounds were identified by their GC retention time relative to known compounds and by comparison of their mass spectra with those present in IIIM library. The GC-MS spectra of the oily fraction of toluene extracts of Piper nigrum unveiled the presence of following components.
Table 1: Different components from cold toluene extract of Piper nigrum
| Compound number | RT(min) | Peak name | Area | Amount/Rf |
| 1 | 5.934 | Toluene | 3.929e+6 | 99.216 |
| 2 | 38.357 | Caryophyllene | 10878 | 0.275 |
| 3 | 41.815 | β–bisabolene | 2341 | 0.059 |
| 4 | 42.325 | δ –Cadinene | 1828 | 0.046 |
| 5 | 43.562 | Elemol | 2101 | 0.053 |
| 6 | 47.459 | (-)- Cedreanol | 3755 | 0.095 |
| 7 | 47.885 | α- Eudesmol | 905 | 0.023 |
| 8 | 63.175 | Piperine | 9249 | 0.234 |
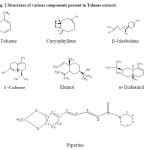 |
Figure 2: Structures of various components present in Toluene extracts. Click here to View figure |
Antimicrobial activity
Cultures
Gram positive bacteria: Bacillus subtilis, Staphylococcus aureus; Gram negative bacteria: Escherichia Coli, Pseudomonas aeruginosa, Salmonella typhi and Fungi Aspergillus niger were obtained from Biotech Research Laboratory, Lovely Professional University.
Chemicals
Nutrient agar and nutrient broth for bacterial cultivation; Potato dextrose agar and potato dextrose broth for fungal cultivation and standard antibiotic like gentamicin were purchased from Hi Media Laboratories Pvt. Ltd., Mumbai.
One gram of the extracts was dissolved in same solvent in such a way that the final concentration of each extract would be 1gm/ml of respective solvent.
Disc diffusion method
The in vitro antimicrobial activity of toluene extracts of pepper were checked by disc diffusion method [10]. Bacterial culture in log phase was inoculated in nutrient agar and plated. The 5 µl of various extracts were poured on to different discs prepared from whatman No: 1 filter paper. The 2 or 3 discs were then placed on the petriplates containing cultures and incubated bacterias for 24 hours at 37⁰C and fungi in B.O.D incubator for 7 days. The diameter of zone of inhibition was measured.
Table 2: In vitro antibacterial activity of Petroleum ether Extracts
| Bacterias | Zone of inhibition |
| Gram (+) Bacillus subtilis | (+) 3mm dia |
| Gram (-) Escherichia coli | (-) |
| Gram (-) Pseudomonas aeruginosa | (-) |
| Gram (+) Staphylococcus aureus | (-) |
| Gram (-) Salmonella typhi | (-) |
Table 3: In vitro antifungal activity of toluene extracts
| Fungus | Zone of inhibition |
| Aspergillus niger | (+) 5 mm dia |
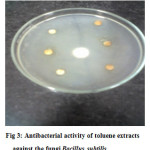 |
Figure 3: Antibacterial activity of toluene extracts against the fungi Bacillus subtilis. Click here to View figure |
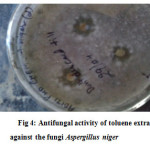 |
Figure 4: Antifungal activity of toluene extracts against the fungi Aspergillus niger. |
Conclusions
The results suggest that the toluene extracts is found very much effective against Bacillus subtilis, and fungus Aspergillus niger. No inhibition zone is seen against the bacteria E.coli, Staphylococcus aureus, Salmonella typhi and Pseudomonas aeruginosa.
Acknowledgements
The author is thankful to IIIM Jammu for GC-MS, NMR, Mass spectra and Lovely Professional University for UV, IR and other lab facility.
References
- Reshmi S.K, Sathya E, Devi P.S. J. of Medicinal Plants Research, 4(15), 1535-1546(2010).
- Siddiqui B.S, Gulzar T, Begum S, Afshan F, Sattar F.A. Natural product research, 19(2), 143-150(2005).
- Parthasarathy U, Asish G.R, Zachariah T.J, Saji K.V, George J.K, Jayarajan K, Mathew P.A, Parthasarathy V.A. Current Science, 94(12), 1632-1635(2008).
- Francois T, Michael J.D.P, Lambert S.M, Ndifor F, Vyry W.N.A, Henri A.Z.P, Chantal M. African J. of Biotechnology, 8(3), 424-431(2009).
- Scott I.M, Jensen H.R, Philogene B.J.R., Arnason J.T. Phytochemistry reviews, 65-75(2008).
- De S, Dey Y.N, Ghosh A.K. Int. J. on pharmaceutical and biomedical research, 1(15), 150-157(2010).
- Berger S, Sicker D. Classics in spectroscopy(2009).
- Madhavi B.B, Nath R.A, Banji D, Madhu M.N, Ramalingam R, Swetha D. Int. J. of Pharmacy and pharmaceutical science, 1(2), 156-161(2009).
- Ikan R. Natural products : a laboratory guide, 2nd edition, 236-237(1991).
- Elgavyar M, Draughon F.A, Golden D.A, Mount J.R. J Food Prot., 64(7), 1019-1024(2001).

This work is licensed under a Creative Commons Attribution 4.0 International License.









