Direct Spectrophotometric Determination of Nickel (II) with o-Chlorophenylazo-bis-acetoxime
Rehana Khanam1*, Saba Khan1 and Rekha Dashora2
1Department of Chemistry, Vidya Bhawan Rural Institute, Udaipur - 313 001, India.
2Department of Chemistry, M.L. Sukhadia University, Udaipur - 313 001, India.
Corresponding Author E-mail: rkhanam2009@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/290230
A new reagent o-chlorophenylazo-bis-acetoxime has been used for spectrophotometric determination of Ni (II). Nickel forms a 1:2 (Ni: R) ethanol soluble greenish yellow complex with the reagent. The working wavelength was taken at 370 nm (ε-2451) in the pH range 7.3-7.9. Beer’s law obeyed between 0.117 - 0.939 ppm and Sandell’s sensitivity is 23.09 ng/cm2.The values of log β found from the two different methods were 8.95 and 8.76, respectively. Interference of 24 diverse ions has been examined.
KEYWORDS:(spectrophotometry; o-chlorophenylazo-bis-acetoxime; nickel (II) ; hydroxytriazene)
Download this article as:| Copy the following to cite this article: Khanam R, Khan S, Dashora R. Direct Spectrophotometric Determination of Nickel (II) with o-Chlorophenylazo-bis-acetoxime. Orient J Chem 2013;29(2). |
| Copy the following to cite this URL: Khanam R, Khan S, Dashora R. Direct Spectrophotometric Determination of Nickel (II) with o-Chlorophenylazo-bis-acetoxime. Orient J Chem 2013;29(2). Available from: http://www.orientjchem.org/?p=22175 |
Introduction
Arylazo-bis-acetoximes are similar to hydroxytriazenes. Although a number of studies related to nickel (II) determination using hydroxytriazenes as reagents have been done1-4, no study has yet appeared on arylazo-bis-acetoximes. Thus, in the present communication of our earlier studies a new reagent o-chlorophenylazobis-acetoxime has been used to determine Ni (II) spectrophotometrically.
Experimental
Synthesis of o-chlorophenylazobis-acetoxime
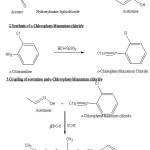
The preparation of o-chlorophenylazobis-acetoxime was carried out in three steps.(Scheme:1)
Synthesis of Acetoxime
Hydroxylamine hydrochloride(13.8 gm) was dissolved in minimum quantity of water. Aqueous solution of sodium hydroxide (8 gm) was then added to it. The solution was then cooled in ice water and acetone (14.7 mL) was then added slowly with stirring for a few minutes. Acetoxime separates out as white crystalline solid. It was then filtered and used for coupling.
Diazotisation of o-chloroaniline
To 0.10 mole of o-chloroaniline, 24.7mL hydrochloric acid was added. The o-chlorophenyldiazonium chloride salt thus formed was dissolved in minimum quantity of water. Maintaining temperture below 5°C, aqueous solution of sodium nitrite (7gm) was then added slowly and drop wise, under mechanical stirring. Diazotised product is then used for coupling.
Coupling of Acetoxime and o-Chlorophenyldiazonium chloride
Freshly prepared acetoxime was dissolved in 150mL of 10% sodium hydroxide solution and then cooled to 0°C by adding crushed ice to it and surrounding the beaker externally with ice-salt mixture. A solution of o-chlorophenyl diazonium chloride was then slowly added under mechanical stirring to this solution. The pH was always maintained close to 10 and the temperature during the entire course of reaction being kept close to 0°C.A dark brown coloured granular product was formed. This was filtered under suction, washed with petroleum ether (40°C-60°C) to remove the coloured impurities and dried. It was crystallized twice from ethanol. During the crystallization process heating was done very slowly as rapid and prolonged heating decompose the compound. C H N analysis corroborated the purity of compound. The compound was subjected to IR spectral analysis and following bands were observed. IR (KBr) cm-1: 3290 (O-H str.), 3078 (C-H str. Ar), 2981 (C-H str., CH3), 1632 (N=N str.), 1419 (N-N str.).The spectra showed the compound to be in pure state. IR spectra (KBr) were recorded on FT IR RX1 Perkin Elmer Spectrometer. Physical characteristics of o-chlorophenylazo-bis-acetoxime are given in Table 1 and the structure of o-CPABA has been given in Figure-1.
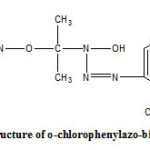 |
Figure 1: Click here to View figure |
 
Table 1: Elemental analysis of o-chlorophenylazo-bis-acetoxime (o-CPABA).
| Molecular Formula of (o-CPABA) | Molecular Weight | M.P.ºC | Elemental Analysis | |||
| C12H17N4O2Cl | 284.50 | 70 | C% | N% | H% | |
| Th. | 50.62 | 19.68 | 6.02 | |||
| Exp. | 50.18 | 19.40 | 5.95 | |||
Spectrophotometric Determination Of Nickel (Ii) Complex With O-Cpaba
Reagent solution of desired concentration was prepared by dissolving its requisite amount in ethanol. Nickel (II) solution of desired concentration was prepared by dissolving the requisite amount of nickel chloride hexa hydrate (A.R.) in double distilled water in the usual way and standardised complexometrically with EDTA of corresponding concentration using murexide as an indicator5. The desired pH of the complex was adjusted with the help of Tris buffer (1%w/v) and perchloric acid (1%v/v). A systronics 106 spectrophotometer was used for absorbance measurements and water proof pH Scan2 tester was used for the pH measurements.
Absorption spectra: Under the optimum condition (pH=7.3-7.9) and five fold excess of the reagent the absorption spectra of nickel complex was taken. Working wavelength was chosen at 370 nm. Subsequent measurements were made at this wavelength using reagent as a blank. Colour development was instantaneous and all the solutions were made up to desired volume with ethanol.
The composition of the complex was determined by two methods Job’s method6 and Yoe and Jones method7 The composition of the nickel complex has been found to be 1:2 (Ni: R) by both of two methods. Beer’s law is obeyed in the entire concentration range studied i. e. 0.117 – 0.939 ppm of nickel. The molor absorptivity and Sandell’s sensitivity values are 2541 and 23.09 ng cm-2, respectively, at 370 nm. Standard deviation ‘s’ was found to be ± 0.031 for 11.74 ppm of Ni (II), (10 determinations).
Solid Complex Of Nickel (Ii) Complex With O-Cpaba
It has been established that Ni (II) forms instantaneously dark green complex with o-chlorophenylazo-bis-acetoxime(o-CPABA) in alkaline medium (pH=7.3-7.9).The composition of Nickel complex with o-CPABA has been established by Job’s and Mole Ratio methods. The composition of Ni (II) complex has been found to be 1:2(Ni: o-CPABA). The requisite quantity of o-chlorophenylazo-bis-acetoxime(o-CPABA) (2.845gm) was dissolved in minimum quantity of ethanol. Similarly requisite quantity of A.R. grade NiCl2.6H2O (1.188gm) was dissolved in double distilled water. pH of the Nickel solution as well as o-CPABA solution were adjusted in the range 7.3-7.9 by using tris buffer or perchloric acid. Both the solution was warmed. This was followed by addition of hot solution with continuous mechanical stirring. The ratio in which o-CPABA and Nickel were mixed was 1:2 (Fe: o-CPABA). After complete addition of Nickel solution, the reaction mixture was further stirred for five minutes and kept aside for ten minutes. The green complex formed was filtered under suction and washed with double distilled water to remove un-reacted metal ion. It was then dried and recrystallised with ethanol. Complex formation has been verified by elemental and spectral analysis. Physical and analytical data are given in Table 2. The tentative structure of 1:2 complex of Ni (II) with o-CPABA has been given in Figure 2
 |
Scheme 1 Click here to View scheme |
Table2: Elemental analysis of 1:2 complex of Ni (II) with o-chlorophenylazo-bis-acetoxime
| Molecular Formula of Complex | Molecular Weight | M.P.ºC | Elemental Analysis | ||||
| C% | N% | H% | Ni% | ||||
| Ni[C12H16N4O2Cl]2 | 626.16 | 150 | Th. | 46.03 | 17.90 | 5.15 | 9.37 |
| Exp. | 45.99 | 17.81 | 5.09 | 9.30 | |||
Table 3: Values of log β and ΔG by two different methods.
|
Name of method |
Reagent |
Composition of complex |
Conc. of Complex (M) |
Em |
Es |
α |
Kinst |
β |
log β |
ΔG at 27°C Kcal mol-1 |
|
Harvey and Manning’s method
|
o-chlorophenyl azo-bis-acetoxime (o-CPABA) |
1 : 2 |
1×10-4 |
0.252 |
0.175 |
0.306 |
0.1651×10-8 |
6.0553×108 |
8.9590 |
-12.0563 |
|
Purohit’s method
|
o-chlorophenyl azo-bis-acetoxime (o-CPABA) |
1:2 |
4×10-4 |
0.808 |
0.700 |
0.134 |
0.1788×10-8 |
5.6237×108 |
8.7499 |
-12.0120 |
|
1:2 |
2×10-4 |
0.440 |
0.351 |
0.202 |
0.1652×10-8 |
6.0510×108 |
8.7819 |
-12.0559 |
Results and Discussion
Stability constant
The stability constant of nickel complex has been determined by two different methods namely (1) Harvey and Manning’s method8 (using mole ratio curve) and Purohit et al. method9 (using Job’s curve). The log b values obtained from both methods agree well indicating the validity of the methods. Values of log b and ΔG are given in Table-3.
Interference studies: Interference studies in presence of 10, 50 and 100 ppm of following 24 cations and anions were performed – Na (I), K (I), NH4 (I), Mn (II), Ba (II), Pb (II), Cd (II), Ca (II), Mg (II), Co (II), Cu (II), Zn (II), Cl–, Br–, I–, F–, CH3COO–, CO32-, SO42-, NO2–, NO3–, S2O32-,WO42- and C2O42-. It was observed that in the determination of 11.74 ppm Ni (II), 10 ppm of Mn (II), Pb (II), Co (II), Cu (II), Zn (II), and C2O42- ions interfered. Ba (II), Mg (II), S2O32- and CH3COO– ions interfered at 50 ppm. The remaining 14 ions viz Na (I), K (I), NH4 (I), Cd (II), Ca (II), Cl–, Br–, I–, F–,CO32-, SO42-, NO2–, NO3– and WO42- which did not interfere up to 50 ppm level also did not interfere at 100 ppm in the Ni (II) determination. Thus, it was found that nickel (11.74) could be determined of any interfering species present even at100 ppm level.
Conclusion
Thus the present investigation has added a new reagent o-chlorophenylazobis-acetoxime for the spectrophotometric determination of Ni (II). This reagent has been shown to be used even in the presence of 14 commonly interfering species as mentioned in the previous paragraphs even up to 100 ppm level. Its easy synthesis, higher yield and economic method for the preparation further enhance its application as spectrophotometric reagent for nickel (II) determination.
References
- R. Bhatt; A.K. Goswami; M.P.Tyagi and D.N.Purohit, Asian J. Chem. 1993; 5 (4) : 1143 – 1144.
- R.S.Shekhawat; R.S.Shekhawat ; R.Singh; R.S. Chauhan; A.K. Goswami and D.N.Purohit, Asian J. Chem. 2001; 13(2): 783-784.
- S.Kumar ; A.K.Goswami ; and D.N.Purohit , Revs. Anal. Chem. 2003; 22(1): 73-80.
- S.Ressalan and C.S.P.Iyer, Revs. Anal. Chem.2004; 23(3):159.
- AI Vogel,. A text book of quantitative inorganic analysis, 3rd ed. ELBS.
- P.Job, Ann. Chim.1928; X-9, 113.
- J.H.Yoe and A.L.Jones, Ind. Eng. Chem. Anal. Ed. 1944, 16, 111.
- A.E.Harvey and D.L.Manning , J. Am. Chem. Soc. 1952, 74, 4744.
- D.N.Purohit; A.K.Goswami; R.S.Chauhan and S. RessalaN, Asian J. Chem. 1999, 11, 123

This work is licensed under a Creative Commons Attribution 4.0 International License.









