Synthesis, Characterisation and Antimicrobial Screening of Co(II), Mn(II) Ni(II), Cu(II) and Zn(II) Complexes of Schiff Base Ligand
G. Valarmathy, R. Subbalakshmi, R. Selvameena and V. Gomathi
Department of Chemistry, Seethalakshmi Ramaswami College, Tiruchirappalli - 620 002, India.
New Schiff base derived from 3-aminophenol and 2-hydroxy-3-methoxy benzaldehyde in ethanolic media and its complexes with Co(II), Mn(II), Ni(II), Cu(II) and Zn(II) have been prepared. These complexes are characterised by IR, 1H NMR,UV, Elemental analysis and Molar conductance measurements. From the analytical and spectral data, the stoichiometry has been found to be 1:2 for all the complexes. The Schiff base ligand and the complexes have been screened for antimicrobial activity by disc diffusion technique. The activity data showed that the metal complexes have more antifungal and antibacterial activity than the parent ligand.
KEYWORDS:Schiff base; 3-aminophenol; 2-hydroxy-3-methoxybenzaldehyde; Metal complexes; Biological activity
Download this article as:| Copy the following to cite this article: Valarmathy G, Subbalakshmi R, Selvameena R, Gomathi V. Synthesis, Characterisation and Antimicrobial Screening of Co(II), Mn(II) Ni(II), Cu(II) and Zn(II) Complexes of Schiff Base Ligand. Orient J Chem 2013;29(1). |
| Copy the following to cite this URL: Valarmathy G, Subbalakshmi R, Selvameena R, Gomathi V. Synthesis, Characterisation and Antimicrobial Screening of Co(II), Mn(II) Ni(II), Cu(II) and Zn(II) Complexes of Schiff Base Ligand. Orient J Chem 2013;29(1). Available from: http://www.orientjchem.org/?p=25205 |
Introduction
Schiff bases derived from aromatic amines and aromatic aldehydes have a wide variety of applications in many fields eg., biological, inorganic and analytical chemistry.1-5 They are known to exhibit potent antibacterial, anticonvulsant and anti inflammatory activities6. In addition some Schiff bases show pharmacologically useful activities like anticancer7, anti-hypertensive and hypnotic8 activities. Schiff bases are important class of compounds due to their flexibility, structural similarities with natural biological substances and also due to the presence of imine moiety (-N=CH-) which is potential in elucidating the mechanism of transformation and resamination reaction in biological system. These novel compounds could also act as valuable ligands whose biological activity has been shown to increase on complexation. Schiff bases of isatin derivatives have been used to demonstrate a variety of biological activities, such as anti-inflammatory9 , anti HIV10 and anti-depressant activities.
The present paper describes the synthesis and characterization of Co(II), Mn(II), Ni(II), Cu(II) and Zn(II) complexes derived from 3-aminophenol and 2-hydroxy-3-methoxy benzaldehyde. The ligand and its metal complexes are characterized by elemental analysis, IR, 1H NMR, UV and molar conductance measurements. The biological activities are also studied against gram positive and gram negative bacterial and fungi organisms for Schiff base ligand and their complexes. The structure of Schiff base ligand is given in Figure 1.
Experimental
Materials and Methods
All chemicals used were of analytical reagent grade (AR) and of highest purity available . Solvents were purified and dried according to the standard procedures. All metal (II) compounds were used as acetate salts. IR spectra of the complexes were recorded in KBr pellets with a Perkin Elmer RX1 FT-IR Spectrophotometer in the 4000-400cm-1 range. The electronic spectra were recorded in DMF on a Perkin Elmer Lambda 35 spectrophotometer in the 190-1100 nm range. The 1H NMR spectra were recorded on a Bruker 400MHz FT-PMR spectrometer(DMSO-d6). Melting points were determined using melting point apparatus (Elico) and were uncorrected. Conductivity measurements for the complexes were carried out using Elico conductivity bridge and a dip conducitivity cell in dimethyl formamide as solvent.
Synthesis of Schiff base ligand
(L) The Schiff base was prepared by the condensation of equimolar amounts of 3-aminophenol (0.002mol) and 2-hydroxy-3-methoxy benzaldehyde
(0.002mol) in minimum quantity of ethanol. The resulting mixture was then refluxed on a water bath for 4 hours. An orange coloured solid mass separated out on cooling was filtered, washed and dried.The purity of the ligand was checked by melting point, TLC and spectral data. The ligand is insoluble in some common organic solvents viz.acetone, benzene and soluble in polar solvents viz.DMF, DMSO.
Synthesis of metal complexes
Metal complexes were synthesized by mixing the hot solution of ligand (0.004 mole) in minimum quantity of dimethyl formamide and ethanolic solution of metal acetates (0.002 mole). The resulting mixture was then refluxed in a water bath for 6 hours. The complexes obtained in each case were cooled, filtered and washed with ethanol several times to remove any excess of the ligand. Finally complexes were washed and dried.
The micro analytical data, melting point, colour and other physical properties of the ligand and its metal complexes are given in Table 1. The probable structure of the complexes proposed in the present work is given in Figure 2.
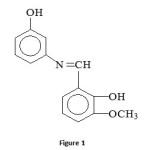 |
Figure 1: Click here to View figure |
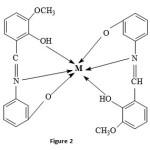 |
Figure 2 Click here to View figure |
Table 1: Physical Characteristics and Microanalytical data of Schiff base ligand and their complexes.
| S.No | Ligand/Complexes | Colour | Molecular Formula | M.P ◦C | Yield % |
Elemental Analysis (%) Calcd(found) C H N |
CN | %M cald(found) | Λmohm-1m2
mol-1 |
||
| 1 | L
|
orange | C14H13NO3 | 140 | 75 | 57.73(58.03) | 4.46(4.40) | 4.81(4.80) | – | – | – |
| 2 | [CoL2] | Pale yellow | C28H22N2O6Co | 250 | 60 | 62.11(62.01) | 4.06(4.03) | 5.18(5.05) | 6 | 10.89(10.5) | 2.60 |
| 3 | [MnL2] | Dark brown | C28H22N2O6Mn | 220 | 65 | 62.57(62.37) | 4.09(3.91) | 5.21(5.20) | 6 | 10.22(9.58) | 2.37 |
| 4 | [NiL2] | Yellow | C28H22N2O6Ni | 221 | 70 | 62.14(62.12) | 4.06(4.01) | 5.18(5.12) | 6 | 10.85(10.2) | 2.94 |
| 5 | [CuL2] | Dark brown | C28H22N2O6Cu | 160 | 55 | 61.59(61.52) | 4.00(4.12) | 5.13(5.09) | 6 | 11.65(11.0) | 2.37 |
| 6 | [ZnL2] | Pale yellow | C28H22N2O6Zn | 150 | 55 | 61.38(61.31) | 4.01(4.02) | 5.11(5.15) | 6 | 11.95(14.3) | 6.02 |
Results and Discussion
The Schiff base ligand is synthesized by using equimolar quantities of 3-aminophenol and
2-hydroxy-3-methoxybenzaldehyde and is complexed with metal acetates. The metal complexes derived vary in their colour. All the complexes are stable, non-hygroscopic and coloured solids. The low molar conductance values in the range of 2.3 – 6.0 ohm-1cm2mol-1 in Co(II), Mn(II) , Ni(II), Cu(II), and Zn(II) chelates indicate that they are non-electrolytic in nature 12.
Infrared Spectra
The infrared spectral data of the Schiff base and its metal complexes are recorded in Table-2. Schiff base showed a strong absorption band at 1603 cm-1 characteristic of ν (C=N) whereas the broad band at 3474 cm-1 characteristic of hydrogen bonded ν(O-H) stretching vibration 13. The azomethine ν(>C=N) band at 1603 cm-1 in Schiff base is shifted to lower frequency in Co(II), Mn(II),Ni(II),Cu(II), and Zn(II) by 6,11,5,109 and 3cm-1 respectively which indicated the co-ordination of azomethine nitrogen on complexation 14. The disappearance of phenolic ν(OH) at 3474 cm-1 in all the complexes suggests the coordination of phenolic oxygen after deprotonation15. The linkage with oxygen atom is further supported by the appearance of a band in the region around 702 cm-1 which may be assigned to ν (M-O)16. A further evidence of the coordination of the Schiff base with the metal atom was shown by the appearance of a new weak frequency band at 533-560cm-1 assigned to the metal nitrogen ν(M-N)17. These new bands were observed only in the spectra of the metal complexes and not in Schiff base confirming the participation of the donor groups.
Table 2: IR and Electronic spectral data.
|
Ligand/ Complexes |
IR spectral data , cm-1
|
Electronic spectral data, cm-1
|
|||
|
L |
3474 |
1603 |
– |
– |
47431,43767,32235 |
| [CoL2] |
3423 |
1609 |
559 |
741 |
32130,27392 |
| [MnL2] |
3421 |
1614 |
558 |
740 |
30032,36182 |
| [NiL2] |
3427 |
1608 |
468 |
771 |
36212,30521,26126 |
| [CuL2] |
3418 |
1712 |
533 |
702 |
26130 |
| [ZnL2] |
3410 |
1606 |
541 |
732 |
31225 |
Electronic Spectra
Electronic spectrum of the ligand shows two high intensity bands at 47431 and 32235 cm-1 indicating n → n* and π → π* transitions respectively of the ligand moiety 18. The electronic spectra of Co(II) complex displays bands at 32130 and 27392 cm-1. The two bands corresponds to 4T1g(F) → 4A2g(F) , 4T1g (F) → 4 T1g(P) suggesting octahedral geometry of this complex19. Ni(II) complex shows absorption bands at 36212, 30521 and 26126 cm-1. The high intensity band at 36212cm-1 is relatively attributed toL → M charge transfer transitions 20 whereas at 30521 and 26126cm-1 may be due to3A2g 3T1g and
4T1g → 4T2g(P). The Cu(II) complex displays a band at 26130cm-1. This band corresponds to 2E(g) → 2T2g .The electronic spectrum of the Mn(II) complex shows two bands at 36182 and 30032 cm-1 assignable to M → L charge transfer transitions and the 30032cm-1 is due to
6A1g → 4Eg(D)21. Zn(II) complex displays single absorption band at 31225cm-1. This is due to Ligand → Metal charge transfer spectra.22,23
1H NMR Spectra
The 1H NMR Spectra of Schiff base and its complexes were recorded in DMSO (d6). The azomethine proton (-CH=N-) in Schiff base appeared at δ = 8.8 ppm has been shifted to downfield in metal complexes. This confirms the coordination by azomethine nitrogen 24. The aromatic protons in Schiff base appeared in the range at δ 6.93-8.0 ppm and in metal complexes in the range δ 6.54-8.75 ppm 25. The disappearance of phenolic –OH proton signal at δ 13.2ppm confirms the coordination by phenolic oxygen to metal ion.
Antimicrobial activity
Antibacterial and antifungal activity of Schiff base ligand and its cobalt, nickel,copper, manganese and zinc complexes have been tested by disc diffusion technique 26,27. The various gram positive and gram negative bacterial organisms such as Gram negative bacteria pseudomonas aeruginosa, E.coli Gram positive bacteria staphylococcus aureus, Bacillus subtilis and fungi aspergillus niger and candida albicans were used to find out the antimicrobial and antifungal activity. (Table 3). Filter paper discs of diameter 6mm were used and the diameters of zones of inhibition formed around each disc after incubating for a period of 72 hours at 25-30⁰ C were recorded . Results were compared with standard drug Ciprofloxacin for bacteria and Nystatin for fungi at the same concentration. All the new complexes showed a remarkable biological activity against bacteria and fungus. From the results it is clear that the metal complexes are found to have more biological activity than the parent ligand.
Table 3: Antimicrobial Activity of Schiff base ligand and complexes.
|
Antimicrobial activity of the ligand and complexes |
Staphylococcus aureus | Bacillus subtilis | E.coli | Pseudomonas aeruginosa | Candida albicans | Aspergillus niger |
|
Ligand (L) |
++ |
++ |
++ |
++ |
++ |
++ |
|
[CoL2] |
++ |
++ |
+++ |
+++ |
++ |
+++ |
|
[MnL2] |
++ |
+++ |
++ |
+++ |
+++ |
+++ |
|
[NiL2] |
++ |
+++ |
+++ |
++ |
+++ |
++ |
|
[CuL2] |
+++ |
+++ |
+++ |
++ |
+++ |
+++ |
|
[ZnL2] |
++ |
++ |
+++ |
+++ |
+++ |
++ |
Standard= ciprofloxacin 5 g/ disc for bacteria ; Nystatin= 100 units/disc for fungi.
Highly active = +++ (inhibition zone > 15mm) ; Moderatively active = ++ (inhibition zone> 10mm) ; slightly active = + (inhibition zone >5mm); Inactive = — (inhibition zone <5mm)
Conclusion
On the basis of the results obtained from elemental analysis, infrared spectra, electronic spectra, 1H NMR and conductance measurements, an octahedral structure has been proposed for all complexes.
Acknowledgements
The authors express their gratitude to The Secretary, Seethalakshmi Ramaswami College,Tiruchirappalli, India for providing laboratory facilities and the faculty members, PG and Research Department of Chemistry, Seethalakshmi Ramaswami College for their timely help.
References
- Cimerman Z,Miljanic C and Galie N, Croatica Chemica Acta, 73(1),81-95 (2000).
- Singh P, Goel RL and Singh BP, J.Indian Chem.Soc,52,958(1975).
- Perry BF, Beezer AE, Miles RJ, Smith BW, Miller J and Nascimento MG, Microbois,45,181(1988).
- Elmali A, Kabak M and Elermann Y, J. Mol. Struct.,151,477(2000).
- Patel PR, Thaker BT and Zeles, Indian J. Chem., 38A,563 (1999).
- Shah S, Vyas R and Mehta R.H ,J.Ind.Chem Soc., 69,590(1992).
- Pandeya S.N., Sriram. D ,Nath G and Clereq E , Eur. J. Pharma Soc,9:25,25-31(1999).
- More PG, Bhalvankar RB, Patter SC, J. Ind.Chem Soc,78:474(2001).
- Bhattacharya SK, Chakrabarti S, Indian J Exp Bio, 36-118-21 (1998).
- Pandeya S.N., Sriram. D ,Nath G and Clereq E , Eur. J. Pharma Soc,9:25,25-31(1999).
- Singh Gs, Singh T,Lakhan R, Indian J Chem, 36B:951(1997).
- W.J.Geary, Co Ord.Che,. Rev.,81(1971)
- L.N.Sharda and M.C.Ganokar, Indian J. Chem.,27A,617 (1988).
- V.Chinuusamy and K.Natarjan, Synth.React.Inorg.Met.Org.Nanomet.Chem.,23,889(1993).
- V.L.Chavan and B.H.Mehta, Asian J. Chem.,22,5976-5980,8(2010).
- J.H.Deshmukh and M.N.Deshpande, Asian J. Chem., 22,5961-5970,8(2010).
- Nakamoto K, Infrared Spectra of Inorganic and Coordination Compounds,Wiley InterScience, New York,2nd Ed,(1970).
- B.P.Ghargava, R.Bembi and M.Tyagu, J.Indian Chem.Soc, 60,214(1983)
- R.C. Maurya, P. Patel, Specr.Lett.,32,213-236,(1999).
- R.C. Maurya, P. Patel, Specr.Lett.,32,213-236,(1999).
- J.D.Lee, Concise Inorganic Chemistry, 5th Edition, Blackwell Science Publishers, p967, Reprint (1999).
- ABP Lever, Inorganic Electronic Spectroscopy, Elsevier, Amsterdam (1984).
- Zahid H.Chohan, Muhammed M.Naseer, Applied OrganoMetallic Chemistry, 21,728-738,(2007)
- HR. Singh ,B.V. Agarwala , J. Indian chem..Soc., 65, 591-593,(1988).
- B.V.Agarwala, S. Hingorani, V. Puri, CL Khetrapal , GA Nagangowda , Transition Met. Chem., 19, 25-27,(1994).
- A. Rahman, M.I.Choudhary, W.J. Thomsen, Bioassay Techniques for Drug Development, Harwood Academic Publishers, Netherlands,(2001). Indian Pharmacopoeia, IIA, 105 (1996)

This work is licensed under a Creative Commons Attribution 4.0 International License.









