Stigmasterol-3-O-Β-D-Arabinopyranosyl(1®4)-O-Β-D-Glucopyranoside from the Roots of Limonia Crenulata (Roxb.)
Archana Shrivastava 1, Sangeeta Parihar2 and Raina Jadhav3
1Department of Enginering Chemistry, GIIT College, Gwalior, India.
2Department of Chemistry Jodhpur National University, Jodhpur, India.
3Department of Chemistry, IPS Academy, Indore, India.
The methanol soluble part of the concentrated ethanolic extract of defatted roots of Limonia crenulata (Roxb) when worked up phytochemically yielded a saponin which on various chemical reactions and spectral analysis was identified as; Stigmasterol-3-O-β- D-arabinopyranosyl (1→4)-O-β-D-glucopyranoside.
KEYWORDS:Stigmasterol -3 - O - β - D - arabinopyranosyl (1→ 4) - O - β - D -glucopyranoside from the roots of Limonia crenulata (Roxb)
Download this article as:| Copy the following to cite this article: Shrivastava A, Parihar S, Jadhav R. Stigmasterol-3-O-Β-D-Arabinopyranosyl(1®4)-O-Β-D-Glucopyranoside from the Roots of Limonia Crenulata (Roxb.). Orient J Chem 2013;29(1). |
| Copy the following to cite this URL: Shrivastava A, Parihar S, Jadhav R. Stigmasterol-3-O-Β-D-Arabinopyranosyl(1®4)-O-Β-D-Glucopyranoside from the Roots of Limonia Crenulata (Roxb.). Orient J Chem 2013;29(1). Available from: http://www.orientjchem.org/?p=25135 |
Introduction
The plant Limonia crenulata (Roxb)1 is commonly know as Beli in hindi and belongs to Natural-order Rutaceae It occurs in Western and Southern India, Punjab, N.W. Himalay, Shimla, Kumaon, Bihar, Bengal and Assam. Its roots are purgative, and are reported to be useful for curing colic and cardialagia. The dried fruit of this plant work as antidote to various poisons They function as tonic and diminish intestinal fermentation. They also resist the contagion of small pox along with malignant and pestilent fevers. It’s leave are reported to be useful for curing EPILEPSY.
Material and Mathods
2.5kg of air dried powdered and defatted roots of Limonia crenulata (Roxb) were extracted with hot rectified spirit in a round bottomed flask fitted with a reflux condenser on an electric water bath. The ethanolic extract was filtered while hot under concentrated reduced pressure to get brown viscous mass, which was partitioned with n-hexane, benzene, chloroform, acetone ethylacetate and methanol.
The study of the methanol soluble part
The methanol soluble part was concentrated under reduced pressure to get a brown viscous mass which on addition of excess of solvent ether gave a precipitate which on TLC examination over silica gel G, using solvent system n-butanol acetic acid : water (4:1:5) showed two spots indicating it to be a mixture of two compounds.
Therefore the precipitate was subjected to coloumn chromatography2 over silica gel ‘G’ and eluted with acetone : methanol in different proportions.
Eluates from acetone : methanol (1:1) were of the same Rf value and so combined and subsequently removal of the solvent yielded a homogenous mass (confirmed by the TLC). Therefore it was crystallized from pyridine. It analysed for molecular formula C40H66O10, m.p. 190-192°C and [M+] = 706 (CIMS). It was soluble in methanol, and absolute alcohol but insoluble in petroleum ether and benzene and responded to characteristics tests of steroidal saponin3-7.
Result and Discussion
The presence of OH group(s) in steroidal saponin
KBr
Characteristic bands at Vmax 3755 and 3333 cm-1 of the IR spectrum of the saponin indicated the presence of —OH group(s) in it. The number of hydroxyl (-OH) group(s) were estimated by acetylation of saponin with Ac2O/pyridine when it gave an acetylated product. The percentage of the acetyl group in the acetylated product was estimated by the procedure of Weisenberger8 as described by Belcher and Godbert9 (36.54%), which showed the presence of six —OH groups in it.
The presence of double bond in the steroidal saponin
The presence of double bond in the steroidal saponin was indicated by the fact that a solution of saponin in CCI4 produced yellow colour with tetra nitro-methane10, thus indicating the presence of double bond in the steroidal saponin. This was further confirmed by
KBr
a band at Vmax 1597 cm-1 in the IR spectrum of the steroidal saponin.
The presence of methyl groups in steroidal saponin
KBr
Significant band at Vmax 1404 cm-1 in the IR spectrum of the steroidal saponin indicated the presence of —CH3 group(s) in it. The number of —CH3 groups were estimated by Ziesel method (13.00%), which showed the presence of six —CH3 (methyl) group(s) in it.
Acid hydrolysis of saponin
The saponin was hydrolysed with 6% H2SO4 when the sapogenin precipitated out. It was filtered and washed with water.
The sugar moiety(ies) remained in the hydrolysate and studied chromatographically.
The structural study of sapogein
The sapogenin was subjected to TLC, examination when it was found to be homogenous. The sapogenin analyzed for molecular formula. C29H48O, m.p. 221 — 222°C, and [M+] = 412 (CIMS). It responded positively to all the characteristics colour reactions of steroids11.
Presence of hydroxyl group(s) in the sapogenin
KBr
Characteristic bands at Vmax 3486 and 3310 cm-1 in the IR spectrum of sapogenin indicated the presence of —OH group(s) in it. The acetylation of sapogenin with Ac2O / NaOAc in glacial acetic acid yielded monoacetate of the sapogenin. This mono acetate analysed for molecular formula C31H52O2, m.p 194 — 195°C and [M+] = 454 (CIMS). The presence of acetyl groups(9.56%) were estimated by Weisenberger12 as described by Belcher and Godbert13, which showed the presence of only one acetylable hydroxyl group in the sapogenin.
The position of hydroxyl group(s) in the sapoenin
The sapogenin on Cr2O3/ pyridine oxidation, yielded a ketone, m.f. C29H46O, m.p. 227 — 228°C and [M+] = 410 (CIMS), which responded to positive Zimmerman test14 for C-3 keto group, thereby confirming the presence of hydroxyl group at C-3 and further indicated its nature as secondary in the sapogenin.
The presence and position of double bond(s) in the sapogenin
KBr
The characteristics band at Vmax 1609 cm-1 in the IR spectrum of the sapogenin showed the presence double bond(s) in it. On catalytic hydrogenation with Pd/C, the sapogenin gave a tetra hydro derivative m.f. C29H50O m.p. 224 — 225°C and [M+] = 414 (CIMS), which indicated the presence of two double bond in it.
The presence and position of methyl group(s) in the sapogenin
KBr
The IR spectrum of sapogenin showed band(s) at Vmax 1350 and 1380 cm-1, which indicated the presence of angular methyl group(s) in it. Quantitative estimation of methyl group(s) (22.0%) was done by Ziesels15 methods, which showed the presence of six angular methyl group(s) in the sapogenin. The position of methyl group(s) was established by the study of 1HNMR spectrum. The 1HNMR spectrum of sapogenin showed three proton intensity singlet each at b 0.70, b 0.82 doublets each at b 0.75 J-6.5, b 0.68 J-6.7 and triplet b 0.88 J-5.34, assigned for the position of methyl group at C-18, C-19, C-25, C-26, C-28 and C-29 respectively in the sapogenin.
The study of the sugar hydrolysate of the saponin.
The aqueous hydrolysate obtained after separating the sapogenin was neutralized with BaCO3 and the BaSO4 was filtered off. The filtrate which was found to reduce Fehling’s solution was concentrated to get a syrupy mass.
The concentrated hydrolysate was, subjected to paper chromatography with authentic sugar samples on Whatmann No.1 filter paper using aniline hydrogen phthalate as spraying reagent. The analysis revealed the presence of D-arbinose and D-glucose as sugar moieties (confirmed by CoPC and CoTLC with authentic sugar samples).
The quantitative estimation of sugars
Quantitative estimation of sugars present in the saponin was done by procedure of Mishra and Rao16. It was observed that the two sugars were present in the equimolecular ratio.
Thus, it was concluded that one molecule of the steroidal saponin was made up of one molecule of sapogenin and one molecule each of D-arabinose and D-glucose.
The position of attachment of sugars to the sapogenin
Since there is only one hydroxyl group available for glycoside formation in the sapogenin naturally the sugars must be attached on it, thereby concluding that sugars must be attached at C-3. Thus a tentative structure to the saponin was assigned as below:
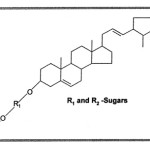 |
Scheme 1 Click here to View scheme |
The sequence of sugars in the steroidal saponin
On graded hydrolysis with 0.04 N H2SO4 for one hour at room temperature the steroidal saponin liberated first D-arabinose followed by D-glucose, thus confirming D-arabinose as terminal sugar.
Thereafter the contents of the flask were subjected to column chromatography over silica gel ‘G’ using chloroform : methanol (1:1) as eluant when a mixture of two prosapogenins were isolated analysed for m.f. C35H58O6, m.p. 219-220 and m.f. C40H66O10, m.p. 239-240 respectively.
The study of the prosapogenin
The prosapogenin analysed for m.f. C35H58O6 which on hydrolysis with 0.04 N H2SO4 gave a sapogenin and D-glucose which was identified Co-PC and Co-TLC, with authentic sample. The sapogenin was identified as stigmasterol by mixed m.p.
The permethylation and hydrolysis
The prosapogenin was subjected to permethylation which was done by the procedure of Khun17, The permetylated prospagenin on hydrolysis yielded 2, 3, 4, 6 tetra — O — methyl — D — glucose (The identity was established by Co-PC and Co-TLC, with authentic sample). This showed that C1 of D-glucose was involved in the glycosidic linkage and also suggested that D-glucose was present in the pyranose form. Thus the prosapogenin was assigned the structure as : Stigmasterol — 3 — O — b— D — glucopyranoside.
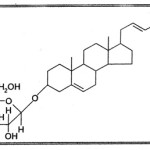 |
Scheme 2 Click here to View scheme |
The study of prosapogenin
The prosapogenin on hydrolysis with 6% H2SO4 yielded a sapogenin which was identified as, stigmasterol. The sugars were identified as D-glucose and D-arabinose (confirmed by Co-PC and Co-TLC with authentic sample).
The permethylation and hydrolysis of prosapogenin
This prosapogenin on permethylation followed by hydrolysis and chromatographic examination of hydrolyrate established the presence of 2, 3, 4, 6 tetra — O — methyl — D — glucose and 2, 3, 4 tri—O—methyl — D — arabinose (by CoPC and Co-TLC with authentic sample) thus indicating that D-arabinose and D-glucose both were present in pyranoside form in it.
The nature of the glycosidic linkage in the saponin
The steroidal saponin18 when subjected to hydrolysis by enzyme almond emulsion liberated the sugars, The examination of sugars by paper chromatography (confirmed by Co-PC and Co-TLC with authentic sample) indicated the presence of D-arabinose and D-glucose thereby confirming the linkage between D-arabinose and the sapogenin as well as between D-glucose and D-arabinose as b in the saponin.
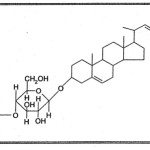 |
Scheme 3 Click here to View scheme |
Conclusion
As such structure to the steroidal saponin was assigned as; stigmasterol — 3 — O —b— D — arabinopyranosyl (1→4) —O — b— D — glucopyranoside.
References
- Chopra, R.N., Nayer, S.L. and Chopra, I.C. “Glossary of Indian Medicinal Plans”, CSIR Publication, New Delhi, p. 119, (1956).
- Peachand Tracey, “Modern Methods of Plant Analysis”, Vol. II, P. 49, (1962).
- Noller, C.R., J. Amer. Chem. Soc., Vol. 64, p. 3047, (1942).
- Tsohugajew, Che. Zig., Vol. 24, p. 542, (1900).
- Mohan Jag, “Organic Spectroscopy Principles and Applications” Narosa Publishing House New Delhi (2000).
- Liebermann, C. “Ber. Deash. Chem. Gas”, p. 1804, (1885).
- Salkwski, E., Hoppeseyler Z., Vol. 57, P. 52, (1908).
- Weisenberger, Microchemic, Vol. 33, p. 51, (1947).
- Belcher, R. and Godbert, A.L.. Semi Micro quantitative Organic analysis, p. 164, (1954),
- Rechard, R.E. and Thomas, N.A., J. Chem. Soc., pp. 368-74, (1974).
- Zeisel, et al, J. Chem. Soc., pp. 2478-82, (1930).
- Weisenberger, Microchemic, Vol. 33, p. 52, (1947).
- Belcher, R. and Godbert, A.L. Semi Micro Quantitative Organic analysis, p. 165, (1954).
- Zimmermann, J. Helv. Chem. Acad., Vol. 26, p. 642-647, (1943).
- Zeisel, et al, J. Chem. Soc., p. 2478, (1930).
- Mishra, S.B., and Rao M.V.K., J. Sci. Ind. Res. Vol. 19, No e, p. 170 (1960).
- Khun, R. Low, I and Trichmann, H., Angew. Chem. Vol. 67, p. 32, (1955).
- Pakrashi, S.P., Indian J. Chem. Vol. 21, p. 468, (1964).

This work is licensed under a Creative Commons Attribution 4.0 International License.









