Reactivity of α-Hydroxy Acids Towards Piperidinium Chlorochromate Oxidation
K. Anbarasu1* and P. Selvi2
1Department of Chemistry, TRP Engineering College(SRM), Tiruchirappalli - 621 105, India.
2Department of Chemistry, Er.PerumalManimekalai College of Engineering, Hosur - 635 117, India.
The kinetics of oxidations of α-hydroxy acids such as glycolic acid, lactic acid and mandelic acids with piperidiniumchlorochromate has been studied in aqueous acetic acid medium. The reaction order is one with respect to oxidant, substrate and fractional order with respect to hydrogen ion concentration. Decrease in dielectric constant of the medium increases the rate of reaction. No appreciable change in the rate is observed by increasing the ionic strength of the medium. The reaction does not induce the polymerization of acrylonitrile. From the kinetic data obtained, the activation parameters have been calculated and plausible mechanism has been proposed. The relative reactivity of the order is α-hydroxy acids viz., glycolic acid < lactic acid
KEYWORDS:Kinetics; Oxidation; Parameters; Piperidiniumchlorochromate
Download this article as:| Copy the following to cite this article: Anbarasu K, Selvi P. Reactivity of α-Hydroxy Acids Towards Piperidinium Chlorochromate Oxidation. Orient J Chem 2013;29(1). |
| Copy the following to cite this URL: Anbarasu K, Selvi P. Reactivity of α-Hydroxy Acids Towards Piperidinium Chlorochromate Oxidation. Orient J Chem 2013;29(1). Available from: http://www.orientjchem.org/?p=25164 |
Introduction
A great variety of new chromium(VI) oxidant together with special reaction conditions have been developed for the chemospecific, regiospecific and stereospecific oxidative generation of functional groups in highly sensitive systems. Recently some neutral or almost neutral chromium(VI) reagents have been developed to effective oxidations under mild conditions. Piperidiniumchlorochromate is found to be a mild selective reagent for the oxidation of alcohols to the corresponding carbonyl compounds1. The oxidation of a-hydroxy acids by various chromium compounds have also been studied extensively2-10. The present work involves the kinetic study of oxidation of a-hydroxy acids with piperidiniumchlorochromate in the presence of perchloric acid.
Experimental
Materials and Methods
Piperidiniumchlorochromate was prepared by literature method1 and its purity was checked by determining chromium(VI) concentration iodometrically. a-hydroxy acids such as glycolic acid, lactic acid and mandelic acids (Aldrich) used were of extra pure variety and their physical constants agreed with literature values. All other chemicals used were of AnalaR grade. Double distilled water can be used for throughout the work.
Kinetic Measurements
The reaction was carried out under pseudo first order conditions and keeping the substrate always in excess. The reactions were followed by monitoring the decrease in absorption of Piperidiniumchlorochromateat 470 nm employing a UV-visible spectrophotometer. Computations of rate constants were made from the plot of log absorbance versus time. All the rate constants given in table 1 are the average of two or more determinations.
Stoichiometry and Product Analysis
The stoichiometric runs were carried out in presence of excess of Piperidiniumchlorochromate at 350C reveal that one mole of oxidant is consumed by one mole of the substrate. The aldo acid is formed as the oxidation product was detected by their characteristic tests11 and IR spectral studies.
The same experimental conditions were used for the kinetic determinations, solutions of oxidant (0.1 mol), glycolic acid (0.1 mol) and perchloric acid (0.1 mol) were mixed and kept under nitrogen atmosphere for 24 h for the completion of the reaction. The main product obtained was found to be the corresponding carbonyl compounds. The oxidation products were detected by their characteristics spot tests11 and IR spectral studies.
Results and Discussion
The kinetics of oxidation of a-hydroxy acids by Piperidiniumchlorochromate(PipCC) was investigated at several initial concentrations of the reactants. The oxidation of glycolic acid by PipCC proceeds smoothly at 313K in 50% acetic acid-water medium. The reaction was found to be first order with respect to PipCC as evidenced by a linear plot of log absorbance versus time. But the rate of reaction is decreased with increase the concentration of oxidant12-14, the plot of [PipCC] versus 1/k1 which is also linear. The rate of reaction is increased with increase the concentration of substrate, the reaction was found to be first order with respect to substrate as evidenced by the unit slope (r = 0.998) of the plot of log k1 versus log [substrate]. It was further well demonstrated by the constancy (Table 1) of the specific reaction rate constant k2. ie., k1/[substrate].
Table 1: Rate Constants for the Oxidation of Glycolic Acids by PipCC at 313 K.
| [PipCC] 102 | [Glycolic Acid] 102 | k1 104 | k2 x 10 |
| (mol dm-3) | (mol dm-3) | (s-1) | (s-1mol dm-3) |
| 0.33 | 0.75 | 11.87 – | |
| 0.44 | 0.75 | 10.62 | – |
| 0.55 | 0.75 | 9.38 | – |
| 0.66 | 0.75 | 8.44 | – |
| 0.77 | 0.75 | 7.39 | – |
| 0.44 | 0.50 | 7.01 | 1.40 |
| 0.44 | 0.75 | 10.62 | 1.41 |
| 0.44 | 1.00 | 14.32 | 1.43 |
| 0.44 | 1.25 | 17.78 | 1.42 |
| 0.44 | 1.50 | 21.25 | 1.41 |
[H+] = 0.68 x 10-1mol dm-3 AcOH : H2O = 50 : 50
At constant concentrations of the reactants, the reaction rate increased with increase in the concentration of the hydrogen ion. But the plot of log k1 versus log [H+] was found to be linear with a fractional slope indicating fractional order dependence with respect to hydrogen ion concentration. Increase in ionic strength of the medium by adding sodium perchlorate has no effect on the reaction rate indicating the involvement of ion neutral molecule in the rate determining step15. The rate of oxidation increases with the increase in percentage of acetic acid. It is well known that as the polarity of the solvent decreases, the rate of oxidation increases. The plot of log k1 versus D-1 is linear with positive slope implying the reactive species in acid medium to be a positively charged one16 and this provides convincing evidence that PipCC is protonated.At constant concentrations of the reactants, the reaction was followed by varying acrylonitrile, [manganous sulphate] while acrylonitrile has no effect, manganous sulphate retarded the reaction rate considerably indicating a two electron transfer mechanism.
Table 2. Rate Constants for the Oxidation of Glycolic Acids by PipCC at 313 K.
| [H+] 10 | AcOH : H2O | k1 104 |
| (mol dm-3) | (v/v) | (s-1) |
| 0.34 | 50:50 | 7.96 |
| 0.68 | 50:50 | 10.62 |
| 1.02 | 50:50 | 13.21 |
| 1.36 | 50:50 | 16.44 |
| 1.70 | 50:50 | 19.81 |
| 0.68 | 40:60 | 7.14 |
| 0.68 | 45:55 | 8.42 |
| 0.68 | 50:50 | 10.62 |
| 0.68 | 55:45 | 12.88 |
| 0.68 | 60:40 | 15.19 |
[PipCC] = 0.44 x 10-2mol dm-3[Glycolic Acid] = 0.75 x 10-2mol dm-3
The rate constants were measured at five different temperatures and activation parameters have been calculated from a plot of log k2 versus T-1 using the Eyring’s equation (Table 3). The low Eaand DH# values supported the proposed concerted mechanism. The liner trend between enthalpies (DH#) and entropies (DS#) of activation (Fig 1)shows that the reaction is controlled by both parameters. The high negatives values of the entropies of activation (DS#) suggest assumption of highly solvated transition state due to its increased polarity. Free energy of activation (DG#) values is nearly constant which indicates that all the a-hydroxy acids are oxidized by the same mechanism.The genuine nature of isokinetic relationship was verified by the Exner criteria by plotting log k303K versus log k313K (Fig 2). This plot is found to be linear with r = 0.999. This linearity of the Exner plot is suggestive of unified mechanism for the PipCC oxidation of all a-hydroxy acids studied.
Table 3. Rate Constants for the Oxidation of Glycolic Acids by PipCC at 313 K.
| [NaClO4] 103 | [MnSO4] 102 | k1 104 | |||
| (mol dm-3) | (mol dm-3) | (s-1) | |||
| 0.00 | – | 10.62 | |||
| 5.01 | – | 10.42 | |||
| 10.02 | – | 10.24 | |||
| 15.03 | – | 10.44 | |||
| 20.04 | – | 10.36 | |||
| – | 0.00 | 10.62 | |||
| – | 2.02 | 8.10 | |||
| – | 4.04 | 8.02 | |||
| – | 6.06 | 8.24 | |||
| – | 8.08 | 8.14 |
[PipCC] = 0.44 x 10-2mol dm-3[Glycolic Acid] = 0.75 x 10-2mol dm-3
[H+] = 0.68 x 10-1mol dm-3 AcOH : H2O = 50 : 50
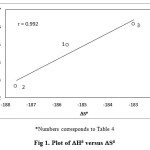 |
Figure 1: Plot of DH# versus DS#
|
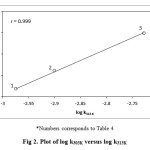 |
Fig 2. Plot of log k303K versus log k313K.
|
Table 4. Rate Constants and Activation Parameters for the Oxidation of a-Hydroxy Acids with PipCC
|
Order k1 104 (s-1) DH# –DS# DG# Ea |
|
Substrate w.r.t. (kJmol-1) (JK-1mol-1) (kJmol-1) (kJmol-1) r |
|
substrate303K 308K 313K 318 K 323K at 313K |
|
Glycolic Acid 1.14 6.82 8.07 10.62 12.64 14.34 12.50 185.57 70.58 15.10 0.992 |
|
Lactic Acid 0.93 8.34 10.47 12.59 15.07 17.89 12.27 187.57 70.98 14.87 0.995 |
|
Mandelic Acid 1.09 12.52 15.41 18.72 22.81 27.19 12.62 182.99 69.89 15.22 0.999 |
[PipCC] = 0.44 x 10-2mol dm-3 [Substrate] = 0.75 x 10-2mol dm-3 [H+] = 0.68 x 10-1mol dm-3 AcOH : H2O = 50 : 50
Mechanism and Rate law
The reaction shows first order with respect to [PipCC], [substrate] and fractional order with respect to [H+]. Increase in ionic strength of the medium by adding sodium perchlorate has no effect on the reaction rate indicating the involvement of an ion and neutral molecule in the rate determining step. The rate of reaction is increased with increase the percentage of the medium. No polymerization was observed with acrylonitrile, indicating the absence of free radicals during the reaction. The retardation of the rate by the addition of Mn2+ ion confirms that a two electron transfer process is involved in the reaction. Based on the above facts, the following mechanism was proposed.(Scheme 1)
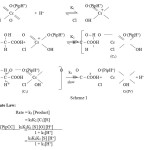 |
Scheme 1: |
Structure and Reactivity of a-Hydroxy Acids
Hydrogen abstraction mechanism is unlikely though not ruled out in view of the failure to induce polymerization of acrylonitrile. The reaction rate increases with the introduction of an alkyl group in the substrate indicating electron deficient transition state. The oxidation rate of mandelic acid is much faster compared to other hydroxyl acids. Stabilization of the intermediate17 may probably be the reason for this behavior.
References
- H.N.Sheikh, M.Sharma and B.L.Kalsotra, J. Indian Chem. Soc., 82, 913(2005)
- P.Aparna, S.Kothari and K.K.Banerji,J. Chem. Res(S), 367(1994)
- S,M,Sundaram, M.Amutha and R.M.Sockalingam, Chem.and Environ. Res., 5(1-4), 229(1996)
- D.Sethumathavan and K.R.Sankaran, Oxid.Commun., 20(4), 587(1997)
- S.MeenakshiSundaram and V.Sathiyendiran, Oxid.Commun., 21(1), 71(1998)
- S.MeenakshiSundaram and V.Sathiyendiran, Oxid.Commun., 21(1), 77(1998)
- R.Gurumurthy and B.Karthikeyan, Oriet. J. Chem., 14(3), 435(1998)
- K.R.Sankaran and C.Anbuselvan, Asian J. Chem., 10(4), 806(1998)
- V.Sundari, S,MeenakshiSundaram and C.Vasuki, Oxid.Commun., 22(3), 400(1999)
- K.G.Sekar and A.Prabakaran, Oxid.Commun., 31(4), 879(2008)
- F.Feigl, “Spot Tests in Organic Analysis”, Elsevier, Amsterdam, 482(1966)
- M.K.Dorfman and J.W.Greyder, Inorg. Chem., 1, 799(1962)
- N.Vekatasubramanian and V.S.Srinivasan, Indian J. Chem., 8, 57(1970)
- M.Krishnapillay and A.Thirunavukarasu, Indian J. Chem.,20B, 583(1981)
- P.Chockalingam, P.S.Ramakrishnan, S.J.Arulraj and K.Nambi, J. Indian Chem. Soc., 69, 247(1992)
- E.S.Amis, “Solvent Effects on Reaction Rates and Mechanism”, Academic Press, Newyork, 40(1966)
- S.D.Paul and D.G.Pradhan,Indian J. Chem., 9, 835(1977)

This work is licensed under a Creative Commons Attribution 4.0 International License.










