Preparation and Structural Studies of Schiff-bases and their Complexes Derived from Bio-potent Metals
Rahul Kumar Rastogi*, Poonam Garg and Shamim Ahmad
Department of Chemistry (Applied Sciences and Humanities),Invertis University Bareilly, India.
Metal chelates of Fe(III),Mn(III) and V(III) with schiff bases have been synthesized by the condensation of 5-nitrobenzaldehyde with hydrazinecarboxamide/thiosemicarbazidehydrochloride and characterized by using physicochemical and spectroscopic methods. The ligands NBALHC and NBALTSC were found to behave in neutral bidentate (N,O/S)manner. The complexes formed by these ligands have been found to have octahedral / square-pyramidal stereochemistry. The ligands and their respective complexes were also screened for their antibacterial activity.
KEYWORDS:Fe(III); Mn(III);V(III);Complexes; Schiff bases
Download this article as:| Copy the following to cite this article: Rastogi R. K, Garg P, Ahmad S. Preparation and Structural Studies of Schiff-bases and their Complexes Derived from Bio-potent Metals. Orient J Chem 2013;29(1). |
| Copy the following to cite this URL: Rastogi R. K, Garg P, Ahmad S. Preparation and Structural Studies of Schiff-bases and their Complexes Derived from Bio-potent Metals. Orient J Chem 2013;29(1). Available from: http://www.orientjchem.org/?p=25211 |
Introduction
Transition metal ions are essential to many biological systems in nature(1). Metal ions are involved in a large number of chemical reactions by virtue of their ability to coordinate to simple or polymeric donor species. Transition metal coordination compounds containing the schiff base ligands have been
interest for many years. These complexes play an important role in the development of coordination chemistry related to catalysis and enzymatic reactions, magnetism and molecular architectures(2-4). Imines are an important class of ligands in coordination chemistry and have various applications in analytical chemistry and in catalysis(5,6) .keeping the above facts in mind, transition metal complexes of schiff bases derived from 5-nitrobenzaldehyde and hydrazinecarboxamide/thiosemicarbazidehydrochloride with Fe(III), Mn(III) and V(III) metal ions are reported.
Experimental
All chemicals used were of analytical or equivalent purity. 5-nitrobenzaldehyde,hydrazinecarboxamide / thiosemicarbazidehydrochloride and metal salts were obtained from Sisco-Laboratories. Distilled solvents were used throughout the experiments. The melting points of all the complexes were determined by open capillary method. Elemental analyses were carried out at RSIC CDRI, Lucknow. The metal content for all the metal complexes were determined as reported in the literature(7). The complexes were examined for solubility using various polar and non-polar solvents. Molar conductivity of ligands and metal complexes were recorded using 1×10-3 M solution in DMF and DMSO on PhilipsConductivityBridge at Department of Chemistry, BareillyCollege, Bareilly. IR spectra were recorded on Perkin-Elmer Spectrometer (RSIC CDRI, Lucknow) using KBr pellets. Electronic spectra were recorded on Beckmann DU-2-Spectrophotometer at Department of Chemistry, BareillyCollege, Bareilly. Magnetic-Susceptibility was measured on a Gouy-balance using CuSO4.5H2O as a calibrant. The analytical data, colour, magnetic moment and important IR spectral bands are recorded in table -no. 1and table no.2 respectively.
Preparation of the Ligands
The schiff bases were prepared by the condensation of carbonyl and amino-compounds. The amino compound was dissolved in ethanol and refluxed for about half an hour. Now, the requisite amount of carbonyl compound was added to the flask and this mixture was refluxed for about 6hr. and kept for 24hr. The crystals of ligand were obtained and purified by recrystallisation. The purity of ligand was checked by elemental analysis and melting point.
Preparation of Metal-Complexes.
Metal complexes were synthesized by refluxation precipitation method. To the hot methanolic solution of ligand, methanolic solution of metal salts was added dropwise from dropping funnel and the resulting mixture was refluxed for 6hr. on a water bath and cooled. The pH of the solution was adjusted. The metal complexes obtained were filtered, washed with water and then with hot methanol and dried in vacuum desiccator.
Results and Discussion
All the complexes are coloured, stable in air and decomposes at higher temperature. The elemental analyses of metal complexes suggest 1:2 metal to ligand stoichiometry. The solid complexes were found to be soluble in DMSO and DMF and the molar conductance was measured at 10-3M dilution (at 25oC). The values of molar conductance in these solvents suggest 1:3 electrolytic nature for Fe(III), Mn(III) and V(III) complexes. Magnetic measurements and the electronic spectra of Fe(III), Mn(III) and V(III) complexes were also obtained in order to have information on their probable geometries.
The Fe(III) complexes show magnetic moment in the range of 4.98-5.20 B.M. which indicates high-spin octahedral geometry corresponding to five unpaired electrons. The electronic spectra of Fe(III) complexes show characteristic bands in the region 16660-17666, 23525-23820, 29400-29880cm-1 corresponding to 6A1g → 4T1g, 6A1g → 4T2g and 6A1g 4Eg → transitions, respectively, which are expected for an octahedral Fe(III) complex (8).
Magnetic moment for Mn(III) complexes lie in the range of 4.80-4.91 B.M. revealing the high spin nature of the complexes, corresponding to four unpaired electrons. This value is indicative of square pyramidal configuration for the Mn(III) complexes (9). The electronic spectra of Mn(III) complexes show an intense charge-transfer band at 27000 cm-1 and two d-d transitions at 19000 –
cm-1 and 13000 cm-1. Since, Mn+3 ion is easily reducible, charge transfer will be from ligand to the metal corresponding to → t2(transition)(10). The other two bands may be assigned to 5B1 → 5B2(13000cm-1) and 5B1 5E (19000cm-1), respectively. These are characteristic of square pyramidal geometry around Mn+3 ion.
The V(III) complexes show magnetic moment in the range 2.73-2.80 B.M. which is very nearly equal to the value expected for d2 system like V+3ion. The value also suggested paramagnetic nature and octahedral geometry for the complex (11). The electronic spectra of the vanadium(III) complexes show weak and broad bands in the range 15800-17260 cm-1 with a shoulder in the range 19980-22730 cm-1. The bands at higher wave numbers were considerably more intense and better resolved. Of the two bands mentioned above, the low-energy band may be assigned to the 3T1g 3T2g transition in pseudo-octahedral symmetry and higher energy band to 3T1g → 3T1g(P)(12).
The characteristic infrared bands(4000-200cm-1)for the free ligands NBALHC / NBALTSC when compared with those of its Fe(III), Mn(III) and V(III) complexes provide meaningful information regarding the bonding sites of the ligands. A red shift in v(C=N)(13) band (1640-1610cm-1) in the spectra of free ligands to lower value( 1600-1560 cm-1) in their complexes is consistent with coordination of the azomethine nitrogen to the central metal ion. This is further confirmed by the non-ligand bands at 530-429 cm-1 which are assignable to v(M-N). The infrared spectrum of the ligand NBALHC shows strong broad band at 1740 cm-1 which may be assignable to v(C=O)(14). In the spectra of the complexes this band is shifted to lower frequency region(1710-1700cm-1) and appearance of non-ligand bands at 390-360 cm-1 assignable to v(M-O) further confirms the coordination of carbonyl oxygen atom to the metal ion. A strong and broad band at 815cm-1 in the free ligand NBALTSC spectrum is due to v(C=S)(15) which has been found to shift to lower frequency region (790-770 cm-1) in the complexes pointing to the coordination of the thione sulfur atom of thiosemicarbazone moiety which is further confirmed by the occurrence of non-ligand bands at 465-430 cm-1 assignable to v(M-S).
The IR spectrum of the complexes thereby excluding the involvement of any other goup in coordination and suggesting bidentate nature of the ligands NBALHC and NBALTSC respectively. The presence of coordinated water molecules is indicated by the appearance of new bands at 3400-3200cm-1 and 840-825cm-1 due to stretching mode of -OH and rocking mode of coordinated water molecules. The TGA also supported the inference of IR spectra. The thermogram shows the loss of two water molecules for Fe(III) and V(III) complexes while loss of only one water molecule for Mn(III) complexes.
Antibacterial Activity
All synthesized compounds were evaluated for their antiproliferative activities by inhibition zone technique(16) against six different gram positive and gram negative bacteria, Escherichia coli, Staphylococcus aureus, Salmonella typhi, Bacillus subtillis, Shigella flexnri and Pseudomonas aeruginose. The results of antibacterial screening have been compared with the conventional bactericide streptomycin taken as standard in each case. It is evident from Table no.3 that although the schiff base NBALHC and NBALTSC alone are quite toxic,their activity is increased upon complexation(17).
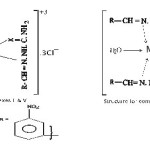 |
Figure 1 |
 
On the basis of available evidences, octahedral geometry may be suggested for all iron(III) and vanadium(III)complexes while square pyramidal geometry is suggested for all manganese(III)complexes.
References
- Ibrahim Y & Alaaddin C,Trans Met Chem, 28, 399(2003).
- S.Goyal and K.Lal, J.Indian Chem.Soc., 66,477(1989).
- B.Dash,P.K.Mahapatra, D.Panda and J.M.Patnaik, J.Indian Chem.Soc., 61,1061(1984).
- R.K.Parashar, R.C. Sharma,A. Kumar and G.Mohan, Inorg. Chim. Acta., 151,201(1988).
- M.Nath and R.Yadav,Bull.Chem.Soc.,(Japan), 70,1331(1997).
- G.Wilkinson,R.D.Gillardand J.A. Mccleverty,Comprehensive Coordination Chemistry,Pergamon Book Ltd, New York,edn.2,Vol.6(1987).
- A.I.Vogel,A Text Book of Quantitative Inorganic Analysis, ElBS-Longman, London, edn.3(1969).
- Narang K K & Singh V P, Synth React Inorg Met Org Chem, 23,971(1993),Lever ABP,Inorganic Electronic Spectros copy, 2nd Edn (Elsevier,Oxford,New York)(1984).
- Ismail A Patel, B T Thaker & P B Thaker, Indian J.Chem., Vol.37A,429-433(1998).
- Sharma B C & Patel C C, Indian J. Chem, 11,941(1973).
- Kamalendu Dey,Bijali Bikash Bhaumik and Saikat Sarkar, Indian J.Chem., Vol.43A, 773-777(2004).
- Machin D J and Murray K S, J. Chem.Soc. A , 1498(1967).
- K.K. Aravindrakshan, Indian J. Chem., 26 A, 291(1987).
- L. J. Bellamy, Advances in Infrared Group Frequencies, Mentham & Co., P.117(1968).
- Joby Thomas and Geetha Parameswaran, Asian J. Chem., 14, 1354(2002).
- Bansal A & Singh R. V, Bol Soc Chil Quim, 45,479(2009).
- Chohan Z H,Praveen M & Ghaffar A, Synth React Inorg Met Org Chem, 28,1673(1998).

This work is licensed under a Creative Commons Attribution 4.0 International License.









