Potentiometric and Thermodynamic Studies of Some Metal Complexes With Carboxy Methyl Mercapto Succinic Acid
K. K. Gupta, Tejinder Kaur and Ekta Dadhich*
P. G. Department of Chemistry, Government College, Kota - 324 001, India.
The metal ligand stability constants of carboxy methyl mercapto succinic acid with some transition metals ions have been determined in dioxane –water mixture at ionic strength µ= 0.1M(KNO3) at three different temperature (25°,35° and 45°C) employing Bjerrum-Calvin pH titration technique. The stability of complexes follows the order Cu2+→ Ni2+→ Mn2+ The free energy, enthalpy and entropy changes involved in the complexation have also been evaluated at 35°C in 40%(v/v) dioxane – water mixture.
KEYWORDS:Free energy; Dioxane; Potentiometry; Stability constant
Download this article as:| Copy the following to cite this article: Gupta K. K, Kaur T. Dadhich E. Potentiometric and Thermodynamic Studies of Some Metal Complexes With Carboxy Methyl Mercapto Succinic Acid. Orient J Chem 2013;29(1). |
| Copy the following to cite this URL: Gupta K. K, Kaur T. Dadhich E. Potentiometric and Thermodynamic Studies of Some Metal Complexes With Carboxy Methyl Mercapto Succinic Acid. Orient J Chem 2013;29(1). Available from: http://www.orientjchem.org/?p=25138 |
Introduction
Mercapto compounds have several application in biological, pharmaceutical and other chemical fields and are well known to form complexes with various metals. Gupta and coworker1-3 have carried out significant investigation on the electrochemical behavior of several biologically active organo-sulphur compounds and their complexation behavior with metals.
Carboxy methyl mercapto succinic acid has coolant additive with corrosion inhibitor and scale preventive4 property. The complex ester of c.m.m.s.a. may be used directly as lubricants5, or may be blended with other mineral or synthetic lubricants and various additive and it is also used as adhesive6, biodegradable bleach –stabilizers for detergents7.In view of wide pharmaceutical and analytical applications of c.m.m.s.a., it seems interesting to study the complexation equilibria of c.m.m.s.a. with some transition metals in dioxane – water mixture. This communication reports the formation , stability constants, and thermodynamic parameters of Ni2+ , Cu2+ ,Mn2+, complexes with c.m.m.s.a. employ potentiometric technique. The log Kstab values determined at 25°,35°,and 45°C by Calvin and Melchior’s extension8 of Bjerrum’s method9.The stability constant have been further determined by correction term10 and Schroder’s convergence formula11.
Material and Method
All the chemicals used for experiment, such as dioxane, potassium nitrate, nitric acid and c.m.m.s.a.. etc were of analytical grade, Double distilled water was used in preparation of various solutions. All the metal ion solutions were prepared in double distilled water and standardized by using conventional procedures12.A carbonate free sodium hydroxide was used as a titrate and standardized against oxalic acid. The pH measurements were carried out with 335-Systronic pH meter (accuracy±0.05 units) using glass and calomel electrode. The electrode system was calibrated by using standard buffer solutions of pH 4.00 ,7.00,9.2. The empirical correction to pH meter reading in dioxane medium was corrected according to Van-Uitert and Hass relation13.The following sets of titrations were performed under nitrogen atmosphere at ionic strength
µ = 0.1 M (KNO3)at temperature 25°,35°,45°C in 40%(v/v) dioxane – water mixtures against 0.1 M NaOH .The temperature were controlled by an electrically maintained thermostat.
(i) Free HNO3 (2.0 X 10 -3 M)
(ii) Free HNO3 (2.0 X 10 -3 M) + Ligand (2.0 X 10 -3 M)
(iii) Free HNO3 (2.0 X 10 -3 M) + Ligand (2.0 X 10 -3 M) + metal ion solution
(4.0 X10 -4).
The log Kstab values determined at 25°,35°,and 45°C by Calvin and Melchior’s extension8 of Bjerrum’s method9.The stability constant have been further determined by correction term10 and Schroder’s convergence formula11.The thermodynamic parameter for binary complex systems were calculated by Gibb’s Helmholtz and Isobar equation15.
Result and Discussion
Identical titration curves were obtained for the different binary system under investigation, according to the sequence described in experimental section. For the sake of brevity only fig (1,2,3) representing formation curves of metal ions have been given.
Metal and ligand stability constant
Calvin and Melchior’s extension of Bjerrum’s9 method was used for determining stability constant of the complexes from potentiometric titration data and their values were further determined by Schroder’s Convergence formula 11 and Correction term method14 the values of stability constants are given in Table-1.
The values of log K1 and log K2 at 25°,35°,and 45°C were read directly from the formation curves at n = 0.5 and n=1.5 (fig-1,2,3). Theses values increases with temperature which shows that higher temperature is favourable for the formation of stable complexes and follow the order
Cu2+ > Ni2+ > Mn2+
Which is in agreement with Irving-Williams order of stability 14
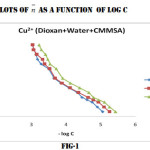 |
Figure 1: Click here to View figure |
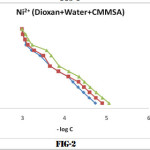 |
Figure 2: Click here to View figure |
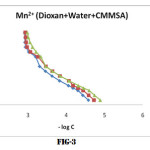 |
Figure 3: Click here to View figure |
Thermodynamic Functions
The values of overall changes in free energy (∆G),enthalpy (∆H) and entropy (∆S) accompanying complex reactions have been determined at 35°C with the help of Gibbs –Helmholtz and Isobar equation15 the values of ∆G, ∆H and ∆S in 40%(v/v)(V/V) dioxane -water mixture are given in Table -2.The negative value of free energy (∆G ) shows that the reaction tends to proceed spontaneously. The values of enthalpy changes are negative indication the exothermic nature of the reaction and the positive values of the entropy changes confirming that the complex formation is entropically favourable.
Acknowledgement
For the research work of “Potentiometric and thermodynamic studies of some metal complexes with carboxy methyl mercapto succinic acid”
The Authors are thankful to Dr.K.K.Gupta Head of Chemistry Department and Shri Sunil Bhargav Principal government college Kota for providing research facilities.
Table 1: Metal Ligand Stability Constant of Complexes of Cu2+, Ni2+ and Mn2+ With C.M.M.S.A. in 40%(V/V) (V/V) Dioxane-Water Mixture at Different Temperature And Ionic Strength µ = 0.1 M (Kno3).
| METALCOMPLEXES | METHOD | TEMPERATURE |
|
a Cu2+ b c Mean Value a Ni2 b c Mean Value a Mn2+ b c Mean Value |
25° 35° 45°logK1 logK2 log ß logK1 logK2 log ß logK1 logK log ß | |
| 4.46 3.17 7.63 4.64 3.32 7.96 4.78 3.47 8.254.39 3.25 7.64 4.57 3.39 7.96 4.72 3.54 8.264.39 3.26 7.65 4.57 3.37 7.94 4.73 3.54 8.274.41 3.23 7.64 4.59 3.36 7.95 4.74 3.52 8.264.32 3.08 7.40 4.46 3.12 7.58 4.60 3.24 7.84
4.24 3.16 7.40 4.40 3.19 7.59 4.59 3.24 7.83 4.34 3.11 7.45 4.47 3.14 7.61 4.63 3.25 7.88 4.30 3.12 7.42 4.44 3.15 7.59 4.59 3.26 7.85 4.06 2.94 7.00 4.18 2.98 7.16 4.32 3.12 7.44 3.95 3.05 7.00 4.09 3.07 7.16 4.22 3.18 7.40 3.94 3.04 6.98 4.08 3.07 7.15 4.21 3.19 7.40 3.98 3.01 6.99 4.12 3.04 7.16 4.25 3.16 7.41 |
||
Method a,b and c represent Bjerrum’s Method, Schroder’s convergence formula and Correction Term Method respectively.
Table 2: Thermodynamic Parameter (∆G), (∆H) and (∆S) Of Complexes Of Cu2+ , Ni2+ And Mn2+ With C.M.M.S.A. At 35°C.
| METAL COMPLEXES | ∆G =KJMOL-1 (-ve) | ∆H= KJMOL-1 (-ve) | ∆S =JMOL-1K-1 (+ve) |
| Cu2+ Ni2+ Mn2+ | 46.88 40.40 21.0444.76 39.63 16.6542.22 38.29 12.76 | ||
References
- Saxena R.S.,Gupta, K.C.and Mittal M., Cand.J.Chem, 46, 311, (1968)
- Saxena R.S.,Gupta, K.C.,Z.Naturforschung, 24 B, 795 (1969)
- Saxena R.S.,Gupta, K.C., Z.Phys.Chem 241,169, (1969)
- Gulley H.J. , US Patent, 4,711,735, (1987)
- Andrew.G.,U.S.Patent, 5,405,545,, (1995)
- Yamada A.and Kimura K., U.S.Patent ,4,196,271,(1980)
- Hatman R., Woodbury P.,,U.S.Patent 5,362,412, (1994)
- Calvin M. and Melchior N.C., J.Am.Chem.Soc, 70 , 3270 (1948)
- Bjerrum J.“metal amine formation in aqueous solution” Hasse P.and son.Copenhagen, 298, (1941)
- Irving H. & Rossotti H.S., J.Chem.Soc, 35,3397, 2904,(1953):(1954)
- Schroder K.N., Acta.Chem, Scand, 20, 1401 (1966)
- Vogel A.I.,Quantitative chemical analysis, 5thedition,Longman,UK.326,(1984)
- Van Uitert L.G.and Haas C.G.J.Am.Chem.Soc, 75(2), 451-457, (1953)
- Irving H. and William R.J.P.,Nature, J.Chem .Soc, 3192-3210,(1953)
- Yatsimirskii K .B. and Vasilev V.P. “ Instability Constants of Complex Compounds’’ Pergamon Press Oxford,New York, 47-52, (1960)

This work is licensed under a Creative Commons Attribution 4.0 International License.









