New Polymeric Hydrogel Formulations Based on Poly(Vinyl Alcohol) for the Controlled Release of Bovine Serum Albumin
Hossein Hosseinzadeh
Department of Chemistry, Payame Noor University, 19395-4697, Tehran, Iran.
Poly(vinyl alcohol) hydrogels have a significant range of mechanical properties based on compositional and processing parameters. Biodegradable hydrogels based on Poly(vinyl alcohol) were prepared for controlled drug delivery of bovine serum albumin. These hydrogels were synthesized by a crosslinking technique using glutaraldehyde as a crosslinker. Hydrogels containing drug were prepared by direct adsorption method. The loading yield was found to depend on both the impregnation time and the amount of encapsulated drug. Glutaraldehyde was utilized as cross-linking agent at different concentration. Increase in the concentration of the cross-linking agent will cause a decrease in the extent of loading of the system.
KEYWORDS:Poly(vinyl alcohol); hydrogel; glutaraldehyde; controlled release
Download this article as:| Copy the following to cite this article: Hosseinzadeh H. New Polymeric Hydrogel Formulations Based on Poly(Vinyl Alcohol) for the Controlled Release of Bovine Serum Albumin. Orient J Chem 2013;29(1). |
| Copy the following to cite this URL: Hosseinzadeh H. New Polymeric Hydrogel Formulations Based on Poly(Vinyl Alcohol) for the Controlled Release of Bovine Serum Albumin. Orient J Chem 2013;29(1). Available from: http://www.orientjchem.org/?p=25125 |
Introduction
Poly(vinyl alcohol) (PVA) is a hydrophilic polymer which is of special interest for the application in medicine owing to its excellent biocompatibility. PVA is one of the most ancient polymers and at the same time the most popular one used in this field. It has been used as a controlled drug delivery system for rectal propranolol, atenalol, indomethacin, phenylpropanolamine and emedastin/HCI [1-3]. Swelling characteristics of this hydrogel depends to the presence of salts and the degree to which the acetate groups are replaced by hydroxyl groups [3]. However, as this hydrogel is quite a hydrophilic system, it releases the drug with a relatively high rate. To prolong drug release from such system, its macromolecular structure should be modified. This can be done by cross-linking and reducing the macromolecular mesh size available for drug diffusion [4].
Increasing the cross-linking density decreases the volume swelling and drug release rate that is attributed to the decrease of diffusion coefficient of drug and solvent. The type of drug release from these systems commonly follows according to Fickian transport.
Hydrogels are gaining increasing popularity in the area of controlled-release drug delivery. Controlled-release technology has rapidly emerged over the past 25 years as a new interdisciplinary science that offers novel approaches to the delivery of bioactive agents including pharmaceutical, agricultural, and veterinary compounds. One of the most important ways to achieve this goal is the use of so-called polymer conjugates which involve small molecules (drugs, etc.) chemically linked to a polymer backbone [5].
Hydrogels can be obtained in various ways. The following methods are most widely employed: chemical cross-linking [6]; mostly using glutaraldehyde as the cross-linking agent [7]; cross-linking by γ radiation [8], by UV radiation [9], and by use of successive freezing/thawing cycles [10]. Intermolecular bonds (mostly hydrogen bonds), which form during the freezing/thawing process of PVA water solutions, act as efficient cross-links.
Eeperimental
Materials
Poly(vinyl alcohol) with molecular weight of 50000 was obtained from Aldrich, Milwaukee, WI, USA and used without further purification. Glutaraldehyde (from Merck) was of analytical grade and used without further purification. The hydrophilic model drugs, bovine serum albumin (BSA) was purchased from Sigma-Aldrich.
Preparation of Hydrogel
Aqueous PVA solution was prepared by dissolving 3.0 g PVA powder in 30 ml deionized water and heating then at 85 °C for 10 h. To the resulting solution, glutaraldehyde as cross-linking agent was added with various concentrations. After 60 min, the reaction product was allowed to cool to ambient temperature. Ethanol (500 ml) was added to the gelled product while stirring. After complete dewatering for 24 h, the hardened gel particles product were filtered, washed with fresh methanol and dried at 50 oC.
Swelling Measurements
An accurately weighed sample (0.20 g) of the powdered superabsorbent with average particle sizes between 40-60 mesh (250–350 mm) was immersed in distilled water (200 mL) and allowed to soak for 3 h at room temperature. The equilibrium swelling (ES) capacity was measured twice at room temperature according to a conventional tea bag (i.e. a 100 mesh nylon screen) method and using the following formula:
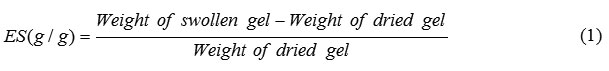
Loading of Drug
Powdered samples (0.5±0.0001 g) with average particle sizes between 40 and 60 mesh (250–350) were accurately weighted and immersed in an acidic solution of BSA (3.0 wt.% BSA solution) at 5oC for 48 h. The swollen hydrogel sample was wiped dry
using laboratory tissue and weighed, then dried to a constant weight at room temperature.
Determination of Loading Efficiency
The amount of drug content entrapped in the hydrogels was determined by an indirect method. After the gel preparation, the washings were collected, filtered with a 0.45 Millipore filter and tested using UV/VIS spectrophotometer (UV-1201, Shimadzu, Kyoto, Japan).
The drugs entrapped exhibited the same λmax as the free drug. This clearly indicates that the drugs entrapped have not undergone any possible chemical reaction during the matrix formation. The difference between the amount of drug initially employed and the drug content in the washings is taken as an indication of the amount of drug entrapped:
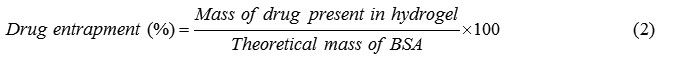
In vitro drug release
The in vitro release of BSA was evaluated using dissolution methodology. In vitro release was carried out in duplicate by incubating 0.1±0.0001 g of the BSA-loaded hydrogels into a cellophane membrane dialysis bag (D9402, SIGMA-ALDRICH) in 50 mL of buffer solution (either pH 1.2 or 7.4) at 37oC. At specific time intervals, 1 mL aliquots of sample was withdrawn through a sampling syringe attached with a 0.45 Millipore filter and after suitable dilution, the concentration of released drug was measured by UV spectrophotometer. The drug release percent was calculated twice using the following equation:

Where L and Rt represent the initial amount of drug loaded and the final amount of drug released at time t.
Result and Discussion
Characterization
One of the most important properties that must be considered is hydrogel microstructure morphologies. Figure 1 shows the scanning electron microscope images of the hydrogel. This picture verifies that the synthesized polymer in this work have a porous structure. It is supposed that these pores are the regions of water permeation and interaction sites of external stimuli with the hydrophilic groups of the hydrogel.
 |
Figure 1: SEM photograph of the hydrogel. Surfaces were taken at a magnification of 500, and the scale bar is 6 μm. Click here to View figure |
Drug Loading efficiency
The amounts of the loaded drug in superabsorbent hydrogels was significantly affected by the impregnation times (Figure 2). It is obvious that with increasing the loading time, the amount of drug loaded is initially increased and then begins to level off. The initial increment in the amounts of the loaded drug with increasing the loading time can be ascribed to the increased drug diffusion into the swollen matrix. The most efficient time of loading efficiency was 6 h, where a major amount of drug was encapsulated.
 |
Figure 2: The dependency of the drug loading amount to the loading time. Click here to View figure |
The effect of the initial concentration of BSA solution on the adsorption capacities of hydrogels is shown in Figure 3. As can be seen from the figure, an increase in drug concentration in the swelling medium increased the amount of adsorbed drug, as observed in many adsorption studies [11-13].
We also investigated the drug loading efficiency of the hydrogels with different crosslinker content (Figure 4). As can be seen, the amount of drug loaded in the hydrogel beads decrease with increasing the content of crosslinker. The increase in crosslink density decreases the swelling of hydrogel, and for that reason the amount of drug loaded into the hydrogel decreases.
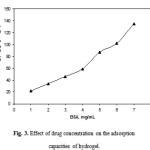 |
Figure 3: Effect of drug concentration on the adsorption capacities of hydrogel. Click here to View figure |
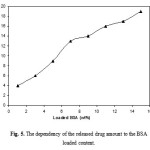 |
Figure 4: Effect of crosslinker content on drug loading. Click here to View figure |
Controlled Drug Release
To determine the potential application of PVA-based superabsorbent containing a pharmaceutically active compound, the drug release behavior form this system under physiological conditions was investigated. The amount of the released BSA from hydrogels was significantly affected by the drug-loaded content (Figure 5). It was generally noticed that there is an immediate release of drug at the time of immersing the sample in the release medium. This is might be due to the surface drug on the hydrogel. This explanation is supported by the fact that this immediate release depends largely on the weight percent of the drug-loaded in the hydrogel. The higher the weight percent of the drug, the higher the percent of the drug immediately released. This immediate drug release is desirable from the practical point of view at the beginning of the applications which must be followed by a period of a controlled release of the drug.
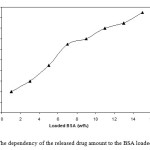 |
Figure 5: The dependency of the released drug amount to the BSA loaded content. |
Conclusion
PVA-based hydrogel was synthesized through crosslinking method using glutaraldehyde as an effective crosslinker. This intelligently superabsorbent network considered as an excellent candidate to design novel drug delivery systems.
The BSA loading and release from the synthesized hydrogels was effective. The drug loading efficiency was decreased with increasing crosslinker content. The release value of BSA from hydrogels was also affected by the drug-loaded amount.
The drug delivery systems based on hydrogels presented in this study may serve as a platform for a wide range of pharmaceutical uses to improve the bioavailability of protein drugs.
References
- Morimoto K., Fukanoki S., Marisoka K., Hyon S .H. and Ikeda Y., Chem. Pharm . Bull. 1989, 37, 2491.
- Morimoto K., Nagayasu A., Fukanoki S., Morisaka K., Hyon S.H. and lkada Y., J. Pharm. Res. 1989, 6, 338.
- Morita R., Honda R. and Takahashi Y., J. Control. Rel., 2000, 63, 297.
- Kim C. and Lee P.I. Pharm. Res., 1992, 9, 10.
- K. Park. Controlled Drug Delivery, Challenges and Strategies, Washington DC, American Chemical Society, 1997.
- Ossipov D. A. and Hilborn J. Macromolecules, 2006, 39, 1709.
- Purss K. H. Qiao G. G., and Solomon, D. H. J. Appl. Polym. Sci., 2005, 96, 780.
- Ajji Z. Radiat. Phys. Chem., 2005, 74, 36.
- Benamer S., Mahlous M., Boukrif A., Masouri B., and Larbi Y. S. Nucl. Instrum. Methods Phys. Res., Sect. B, 2006, 248, 284.
- Hatakeyema T., Uno J., Yamada C., Kishi A., and Hatakeyama H. Thermochim. Acta, 2005, 431, 144.
- Akkas P, Sari M, Sen M, and Guven O. Radiat. Phys. Chem. 1999, 55, 717.
- Saraydın D, Karadag E, Oztop H.N., and Guven O. Biomaterials, 1994, 15, 917.
- Mahkam M, Doostie L, and Siadat S.O.R. Inflammopharmacology, 2006, 14, 72

This work is licensed under a Creative Commons Attribution 4.0 International License.









