An Efficient Oxidation of 1,4-Dihydropyridines by Hydrogen Peroxide in the Presence of Copper(II) Chlorideat at Room Temperature
Farhad Hatamjafari* and Farzad Alijanichakoli
Department of Chemistry, Faculty of Science, Islamic Azad University-Tonekabon Branch, Tonekabon, Iran.
1,4-Dihydropyridines oxidizes to pyridine derivatives by hydrogen peroxide in the presence of Copper(II) chloride as catalyst at room temperature.
KEYWORDS:1,4-Dihydropyridines oxidizes to pyridine derivatives by hydrogen peroxide in the presence of Copper(II) chloride as catalyst at room temperature
Download this article as:| Copy the following to cite this article: Hatamjafari F, Alijanichakoli F. An Efficient Oxidation of 1,4-Dihydropyridines by Hydrogen Peroxide in the Presence of Copper(II) Chlorideat at Room Temperature . Orient J Chem 2013;29(1). |
| Copy the following to cite this URL: Hatamjafari F, Alijanichakoli F. An Efficient Oxidation of 1,4-Dihydropyridines by Hydrogen Peroxide in the Presence of Copper(II) Chlorideat at Room Temperature . Orient J Chem 2013;29(1). Available from: http://www.orientjchem.org/?p=25119 |
Introduction
For the first time 1,4-dihydropyridine synthesized by Hantzsch about one century ago. 1,4-Dihydropyridine ring is the common feature for various pharmacological activities such as antitumours [1], antivirals [2], calcium antagonists [3], antidiabetics [4], and antagonists [5]. Aromatization of 1,4-dihydropyridines (1,4-DHPs) to pyridine derivatives the principal metabolic in biologically activity NADH redox processes [6], an efficient route for synthesis of pyridine derivatives are oxidation of 1,4-DHPs [7]. Consequently, this aromatization reaction continues to attract the attention of many organic and medicinal chemists for the discovery of many of protocols applicable to a wide range of 1,4-DHPs [8]. Many of the reported oxidation procedures either suffer from KMnO4 [9], CrO3 [10], Bi(NO3)3 [11], PCC [12], an so on.
Previously, we have synthesized a number of heterocyclic compounds [13-17]. Although many methods are capable of effecting these oxidations but most of the reported are difficult such as separate from the products, long reaction times and low yields. Therefore, we reported the development of an efficient, a facile method for the aromatization of 1,4-DHPs by hydrogen peroxide in the presence of Copper (II) chloride as catalyst at room temperature (Scheme 1). The hydrogen peroxide [18] was selected as the oxidant and Copper (II) chloride as the catalyst was cheap, environmentally friendly, and easy separation.
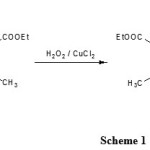 |
Scheme 1: General procedure for oxidation of Diethyl 1,4-dihydro-2,6-dimethyl-4-phenylpyridine-3,5-dicarboxylate. Click here to View Scheme |
Diethyl 1,4-dihydro-2,6-dimethyl-4-phenylpyridine-3,5-dicarboxylate (1 mmol) was dissolved in 20 ml ethanol and Copper (II) chloride (1 mmol) was added to this solution and then 0.2 ml hydrogen peroxide 30% was added in portions over 30 min at room temperature along with stirring of the reaction mixture. The progress of the reaction was monitored by TLC. After recrystallization of the product in ethanol, 96% yield of diethyl 2,6-dimethyl-4-phenylpyridine-3,5-dicarboxylate was obtained.
Spectral data for diethyl 2,6-dimethyl-4-phenylpyridine-3,5-dicarboxylate
Pale yellow solid; m.p.: 60-62 ºC (Ref. [19], 60–61 ºC); FT-IR (KBr): 2976, 1723, 1561, 1230, 1100 cm1; 1H NMR (CDCl3): δ (ppm) = 1.03 (t, 6H, J = 7.5 Hz), 2.66 (s, 6H), 4.03 (q, 4H, J = 7.5 Hz), 7.4-7.8 (m, 5H).
Results and Discussion
Herein, we report oxidation of 1,4-DHPs by hydrogen peroxide and Copper(II) chloride as catalyst was used to pyridine derivatives (Scheme 1), which could provide an efficient, cheap, environmentally friendly, easy separation, high yield and simple route at room temperature for the oxidation of 1,4-DHPs to pyridine derivatives.
Acknowledgements
We gratefully acknowledge the financial support from the Research Council of Tonekabon Branch Islamic Azad University.
References
- Tsuruo T., Iida H., Nojiri M., Tsukagoshi S. and Sakurai Y., Cancer Res., 43: 2905 (1983).
- Krauze A., Germane S., Eberlins O., Sturms I. and Klusa V., Duburs G., Eur. J. Med. Chem., 34: 301 (1999).
- Visentin S., Rolando B., Di Stilo A., Frutterro R., Novara M., Carbone E., Roussel C., Vanthuyne N. and A. Gasco, J. Med. Chem., 47: 2688 (2004).
- Malaise W.J. and Mathias P.C.F., Diabetologia., 28: 153 (1985).
- Poindexter G.S., Bruce M.A., Breitenbucher J.G., Higgins M.A., Sit S.Y., Romine J.L., Martin S.W., Ward S.A., McGovern R.T., Clarke W., Russell J. and Antal-Zimanyi I., Bioorg. Med. Chem., 12: 507 (2004).
- Kill R.J., and Widdowson D.A., In Bioorganic Chemistry., Academic Press New York., 4: 239 (1978).
- Filipan L. M., Litvic M. and Vinkovic V., Tetrahedron., 64: 10912 (2008).
- Balogh M., Hermecz I., Meszaros Z. and Laszlo P., Helv. Chim. Acta., 67: 2270 (1984).
- Vanden E. J., Dorazio R. and Van Haverbeke Y., Tetrahedron., 50: 2479 (1994).
- Geterotsikl K., Chem Abstr., 69: 77095 (1967).
- Mashraqui S. and Karnik M., Synthesis 713 (1998).
- Vanden E. J., Mayence A, and Maquestiau A., Tetrahedron., 48: 463 (1992).
- Azizian J., Hatamjafari F., Karimi A. R. and Shaabanzadeh M., Synthesis., 5: 765 (2006).
- Azizian J., Shaabanzadeh M., Hatamjafari F. and Mohammadizadeh M.R., Arkivoc., (xi): 47 (2006).
- Hatamjafari F., Synthetic Communications., 36: 3563 (2006).
- Azizian J., Hatamjafari F. and Karimi A. R., Journal of Heterocyclic Chemistry., 43: 1349 (2006).
- Hatamjafari F. and Montazeri N., Turkish Journal of Chemistry., 33: 797 (2009).
- Hatamjafari F., Orient. J. Chem., 28: 141 (2012).
- Hashemi M. M., Ahmadibeni Y. and Ghafuri H., Monatshefte fur Chemie 134: 107 (2003).
- Ko K.Y. and Kim J.Y., Tetrahedron Lett., 40: 3207(1999).

This work is licensed under a Creative Commons Attribution 4.0 International License.









