Triton-B Adsorbed on Flysh: An Efficient Support for the Base Catalysed Reactions under Microwave Irradiations
Vijender Goel
Department of Chemistry, Maharshi Dayanand University, Rohtak - 124 001, India
Article Received on :
Article Accepted on :
Article Published : 13 Oct 2016
The application of Triton-B adsorbed on flyash (a waste material of thermal plants ) has been reported for the variety of base catalysed reactions such as synthesis of cinnamic acids by Deobner reaction, synthesis of 1-(2-hydroxyphenyl)-5-phenylpent-4-ene-1, 3-diones by Baker-Venkataraman reaction and synthesis of 3-carboxycoumarins by knoevenagel reaction. This material also acts as a support for the reaction and avoids the use of any solvent in the reaction maintaining the norms of Green Chemistry.
KEYWORDS:Cinnamic acids; 3-carboxycoumarins; 2-cinnamoyloxy acetophenones; 1-(2-hydroxyphenyl)-5-phenylpent-4-ene-1; 3-diones; microwave irradiations
Download this article as:| Copy the following to cite this article: Goel V. Triton-B Adsorbed on Flysh: An Efficient Support for the Base Catalysed Reactions under Microwave Irradiations. Orient J Chem 2012;28(4). |
| Copy the following to cite this URL: Goel V. Triton-B Adsorbed on Flysh: An Efficient Support for the Base Catalysed Reactions under Microwave Irradiations. Available from: http://www.orientjchem.org/?p=22708 |
Introduction
Usually the base catalysed reactions are done in presence of pyridine or piperidine whch are hazardous in nature. The well known example include synthesis of cinnamic acids by condensation of various aromatic aldehydes with malonic acid by Deobner reaction1 under conventional2 as well as under microwave irradiations3. Likewise 3-carboxycoumarins are synthesized using Meldrum’s acid4 by knoevenagelreaction using malanonitrile5.
Herein we wish to report the use of a mild base, triton-B adsorbed on flyash (25% composition) for the base catalysed reactions under microwave irradiations. In recent years, microwave dielectric heating technology combined with solvent free conditions, has been used in many organic reactions, leading to shorter reaction times, higher yields, cleaner reaction products and environmentally more benign conditions compared with the classical heating6,7.
Result and discussion
The main goal of this work is to study the use of a mild base Triton-B adsorbed on flyash for studying a number of base catalysed reactions so as to reduce the reaction time and the use of hazardous reagents. When the same reactions were repeated under ordinary thermal conditions these took much longer time (55-80min) and yields were also not good.
The above said support (Triton-B flyash) has been used for the rapid synthesis of a number of cinnamic acids (la-k). These acids are synthesized by irradiating a mixture of aromatic aldehyde, malonic acid and triton-B adsorbed on flyash (Scheme 1).
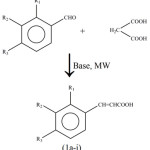 |
Scheme 1 |
Also triton-B has been found useful for the synthesis of a number of 3-carboxycoumarins (2a-d). These coumarins are synthesized by irradiating a mixture of salicylaldehyde, malonic acid and triton-B adsorbed on flyash (Scheme-2).
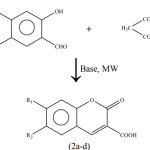 |
Scheme 2 |
This base has further been used successfully for the preparation of 1-(2-hydroxyphenyl)-5-phenylpent-4-ene-1, 3-diones (3a-e), the compounds required for the synthesis of naturally occurring chromone derivatives with a styryl (2-phenylethenyl) or a dihydrostyryl (2-phenylethyl) substituent at 2-position, by Baker-Venkataraman rearrangement. This rearrangement is generally carried out by heating 2-cinnamoyloxyacetophenones with pulverized potassium hydroxide in pyridine medium. Herein we wish to report their synthesis in a simple way by irradiating 2-cinnamoyloxyacetophenones (the esters of cinnamic/p-methoxycinnamic acid with substituted o-hydroxyacetophenones) in the presence of triton-B adsorbed on flyash (Scheme-3).
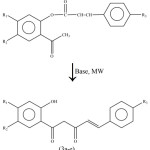 |
Scheme 3 Click here to View scheme |
The structure of all the products have been confirmed by the elemental analysis as well as by their IR and NMR spectra. The results are reported in Table 1.
Table 1: Characterization data, reaction time and yields of compounds (1a-k), (2a-d) and (3a-e) under microwave irradiations and classical methods.
| Compd | R1 | R2 | R3 |
Method A |
Method B |
M.p. | lit MP
(oC) |
|||
| Time
(sec) |
Yield
(%) |
Time
(min) |
Yield
(%) |
|||||||
| 1a | H | H | H | 30 | 97 | 55 | 70 | 133 | 1338 | |
| 1b | H | H | CH3 | 45 | 96 | 55 | 60 | 196-97 | 195-2038 | |
| 1c | CH3 | H | H | 45 | 94 | 60 | 65 | 179-80 | 185-868 | |
| 1d | H | H | Cl | 50 | 93 | 70 | 60 | 248-49 | 249-508 | |
| 1e | H | H | OCH3 | 50 | 94 | 75 | 70 | 171 | 1728 | |
| 1f | H | H | NO2 | 50 | 96 | 75 | 60 | 273 | 2808 | |
| 1g | H | H | OH | 55 | 96 | 70 | 70 | 203 | 2068 | |
| 1h | H | NO2 | H | 60 | 97 | 80 | 75 | 197 | 1978 | |
| 1i | H | OCH3 | OH | 60 | 94 | 80 | 65 | 166 | 1688 | |
| 1j | H | OCH3 | OCH3 | 70 | 96 | 80 | 60 | 178 | 1808 | |
| 2a | H | H | — | 35 | 96 | 55 | 60 | 185 | 186-885 | |
| 2b | H | OH | — | 40 | 94 | 55 | 70 | 280 | 2835 | |
| 2c | H | Cl | — | 50 | 94 | 60 | 75 | 238-39 | 2405 | |
| 2d | OH | H | — | 40 | 95 | 60 | 65 | 246-47 | 248-505 | |
| 3a | H | H | H | 30 | 93 | 60 | 75 | 132-33 | 133-349 | |
| 3b | H | CH3 | H | 45 | 95 | 70 | 75 | 130-31 | 1339 | |
| 3c | CH3 | H | H | 45 | 96 | 70 | 60 | 148 | 1499 | |
| 3d | H | H | OCH3 | 60 | 97 | 75 | 65 | 137-38 | 1409 | |
| 3e | CH3 | H | OCH3 | 60 | 92 | 80 | 65 | 150-51 | 1529 | |
| Method A – Microwave irradiation
Method B – at 100oC on a waterbath |
||||||||||
Experimental
All the melting points were taken in open capillaries and are uncorrected. Homogeneity of compounds was routinely checked on silica gel-G TLC plates using benzene: ethyl acetate (9 : 1) as eluent. The reactions were carried out in a domestic microwave oven (Samsung, Model No. CE118KF, output energy 900W, frequency 2450 MHz with temperature control arrangement) using 30% power for all experiments maintaining the oven temperature at 40oC.
General procedure for preparation of cinnamic acids (la-j)
A mixture of aromatic aldehydes (5 mmol), malonic acid (6 mmol) and triton-B adsorbed on flyash (25% composition) was subjected to microwave irradiation. After the completion of reaction (TLC), the reaction mixture was dissolved in chloroform and filtered to remove the flyash. Distilled off the solvent and added saturated solution of sodium bicarbonate to the residue to remove the unreacted acid. Filtered the solid separated, washed with water, dried and crystallized from alcohol to get the desired product.
General procedure for preparation of 3-carboxycoumarins (2a-d)
Salicylaldehyde (5 mmol), malonic acid (6 mmol) and triton-B adsorbed on flyash (25% composition) was subjected to microwave irradiation. After the completion of reaction (TLC) the reaction mixture was dissolved in chloroform and filtered to remove the flyash. Distilled off the solvent and added saturated solution of sodium bicarbonate to remove the unreacted acid. Filtered the solid obtained, washed with water, dried and crystallized from alcohol to get the desired product.
General procedure for preparation of 1-(2-hydroxyphenyl)-5-phenylpent-4-ene-1, 3- diones (3a-e)
2-Cinnamolyoxyacctophenone (5mmol) and triton-B adsorbed on flyash (25% composition) was subjected to microwave irradiation. After completion of reaction (TLC) the reaction mixture was dissolved in chloroform and filtered to remove the flayash. Distilled off the solvent and the solid separated was filtered, washed with water, dried and recrystallized from alcohol to get the desired product.
Conclusion
The use of triton-B adsorbed on flyash for the synthesis of cinnamic acids, 3-carboxycoumarins and 1-(2hydroxyphenyl)-5-phenylpent-4-ene-1, 3-diones under microwave irradiation proved to be highly efficient and convenient method. This method is superior to conventional heating procedure, where the reaction takes much time (55-80 min) and the yields are significantly lower.
Acknowledgement
The author is thankful to the Head, Department of Chemistry, M.D. University, Rohtak for providing necessary facilities.
References
- Deobner O, Ber, 33, 1900, 2140.
- Koo J, Fish M.S, Walker G.N & Balke J, Org. Synth. Coll. Vol., 4, 1963, 327.
- Mitra A.K, De A & Karchadudhuri N, Synth. Commun., 29(4), 1999, 573.
- Deshmukh M.N, Burud R, Baldino C & Liu J, Synth. Commun., 33(19), 2003, 3299.
- Fringuelli F, Piermatti O & Pizzo F, Synthesis, 15, 2003, 2331.
- Gedey R.N, Smith F.E & Westaway K.C, Can. J. Chem., 66, 1998, 17.
- Varma R.S, Lamture T.B & Varma M, Tetrahedron Lett., 34, 1993, 3209.
- Heilborn I, Cook A, Bunbury HM & Hey D H, Dictionary of Organic Compounds (Oxford University Press, New York) 1956.
- Gaggad M.L, Wadodkar K.N & Ghiya B.J, Indian J. Chem., 24B, 1985, 1244.

This work is licensed under a Creative Commons Attribution 4.0 International License.









