The Chemical Modification of α-Amylase from Locale Bacteria of Bacillus subtilis ITBCCB148 using Citraconic Anhydride
Yandri*, Eka Sulis Sundari, Tati Suhartati and Sutopo Hadi*
Department of Chemistry, Faculty of Mathematics and Natural Sciences, University of Lampung, Bandar Lampung - 35145, Indonesia.
Chemical modification of α-amylase from locale bacteria isolate Bacillus subtilis ITBCCB148 using citraconic anhydride has been performed. increase thermal stability of the enzyme has successfully been done. The results showed that the modified enzymes with citraconic anhydride with modification degree of 45, 73, 82, 83, and 88% have similar optimum pH of 5.5 and optimum temperature of 60°C; ki of 0.030; 0.026; 0.024; 0.021 and 0.018 min-1, respectively; half-life (t1/2) of 23, 27, 29, 33 and 39 minutes, respectively; and ÄGi (in kJ/mol) of 102.91; 103.31; 103.53; 103.9 and 104.33, respectively. The result also showed that the chemical modification on locale bacteria isolate B. subtilis ITBCCB148 using citraconic anhydride has increased the thermal stability of the modified enzymes up to 2-3 times compared to the native enzyme.
KEYWORDS:α-amylase; chemical modification; citraconic anhydride; B. subtilis ITBCCB148
Download this article as:| Copy the following to cite this article: Yandri, Sundari E. S, Suhartati T, Hadi S. The Chemical Modification of α-Amylase from Locale Bacteria of Bacillus subtilis ITBCCB148 using Citraconic Anhydride. Orient J Chem 2012;28(4). |
| Copy the following to cite this URL: Yandri, Sundari E. S, Suhartati T, Hadi S. The Chemical Modification of α-Amylase from Locale Bacteria of Bacillus subtilis ITBCCB148 using Citraconic Anhydride. Available from: http://www.orientjchem.org/?p=11917 |
Introduction
α-Amylase is one of the extracelullar enzymes which potentially can be developed to be applied and used in industrial processes. Industrially, the demand of enzyme especially α-amylase is very high as almost about 25% of the total enzymes sold in the market is α-amylase1. Some industrial sectors which routinely used α-amylase in their process are industries of starch, food, fermented, detergent, textile and paper2.
In order to be used in industrial sector, α-amylase must fulfil some requirements such as thermostability and wider pH range. To obtain the enzyme having high stability and activity at extreme condition may be obtained by direct isolation naturally from extremophilic organism or by chemical modification to enzyme isolated from mesophilic organism3.
According to Mozhaev and Martinek4, chemical modification is one of the way to increase the enzyme stability which soluble in water such as α-amylase. The lysine residue on the enzyme surface causes unstability of the enzyme since it can associate with water around the enzyme. By chemical modification, the lysine structure will be protected by hydrophobic groupd of modifier which will minimize the contact of enzyme with water, so the enzyme will be more stable. present of Chemical modification performed using citraconic anyhidride specifically is able to modify the structure of lysine redidue on the enzyme surface5,6. Yandri et al.7,8 have succesfully done the chemical modification to native α-amylase from locale bacteria isolate B. subtilis ITBCCB148 using dimethyladipimidate and glyoxylic acid where the modified enzymes were increased compared to the native enzyme.
In this paper we reported the chemical modification of α-amylase from B. subtilis ITBCCB148 using citraconic anyhidride.
Experimental
Materials
All chemicals used were the materials with high grade (pro analysis) purity. Locale bacteria isolate B. subtilis ITBCCB148 was obtained from Microbiology and Fermentation Technology Laboratory, Chemical Engineering Department, Bandung Institute of Technology, Bandung, Indonesia.
Research procedure
The following research phases were done: production, isolation, purification, chemical modification and characterization of the native and modified enzymes were based on our previous report9.
Activity test of a-amylase and determination of protein content
Activity of α-amylase was determined based on Fuwa method10 and using dinitrosalicylic acid reagent11. The protein content was determined based on the method by Lowry et al.12.
Chemical modification of the native enzyme using citraconic anhydride13
To 5 mL of native α-amylase (containing 0.008 mg/mL) in 0.1 M phosphate buffer pH 8
was added with citraconic anhydride with volume variation of 20, 30, 40, 50 and 60 μL. pH of the solution was kept constantly at pH 8 by the addition of 0.1 M Na2HPO4 solution. The mixing using magnetic stirrer at room temperature for 1 hour.
Characterization of the native and modified enzymes
The characterizations of the native and modified enzymes including: determination of modification degree, determination of optimum pH and determination of thermal stability.
Determination of optimum pH of the native and modified enzymes
To find the optimum pH of the native and modified enzymes buffer phoshate 0.05 M was used with pH variation of 5.0; 5.5; 6.0; 6.5; 7.0; 7.5; 8.0; 8,5 and 9.0 by keeping the temperature constantly at 60°C.
Determination of optimum temperature the native and modified enzymes
The determination of optimum temperature of native and modified enzymes was done by varying the temperature at 55, 60, 65, 70, 75 and 80°C.
The stability test of the native and modified enzymes
The stability of native and modified enzymes was done based on the known procedure which entailed measuring the residual activity of the enzyme after being incubated for 0,10, 20, 30, 40, 50, and 60 minutes at optimum temperature, where the initial activity of enzyme without heating was given a value of 100%.
Determination of half-life (t½), ki and DGi
Determination of ki value (thermal inactivation rate constant) and the denaturation energy change (DGi) of the native enzyme and the modified enzyme was done using the equation developed by Kazan et al.14.
Results and Discussion
The effect of chemical modification to optimum pH
Figure 1 shows the native α-amylase has optimum pH 6.0, while the modified enzymes with modification degree 45, 73, 82, 83, adn 88% have optimum pH 5.5. The modified enzymes have the wide working pH range of 5.0 – 9.0 especially to the modified enzyme with modification degree of 82, 83, and 88%, while the native enzyme has working pH range of 5.5 – 8.0. The modified enzymes with modification degree of 82, 83, and 88% have better residual activity at pH range of 7.5 – 9.0 than the modified enzymes with modification degree of 45, and 73% and native enzyme.
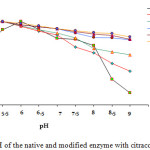 |
Figure 1: Optimum pH of the native and modified enzyme with citraconic anhydride |
Chemical modification can be applied to increase the enzyme stability against pH due to the free NH2 group on the lysine residue exposed to the surface interacted with citraconie anhydride15, as a result there is charge change on the enzyme structure.
The change of optimum pH to 5.5 (acidic) is due to the NH2 group undergo to NH3+ in acidic condition. Since by the modification process most of the NH2 groups will be modified, so in the acidic condition the NH2 group which should change to cation did not occur. This condition caused the modified enzymes were more stable than the native one.
The effect of chemical modification to optimum temperature
Figure 2 shows that there is no change on the optimum temperature to the modified enzyme compared to the native enzyme. However the modified enzymes have better activity at 70 and 80oC compared to the native enzyme. At 70oC the native enzyme has residual activity of 66%, while the modified enzyme with modification degree of 45, 73, 82, 83 and 88% have residual activity of 68%, 69%, 70%, 84% and 86%, respetively. At 80oC the native enzyme has residual activity of 14.5%, while the modified enzymes have residual activity of 28%, 29%, 32%, 38% and 48%, respectively.
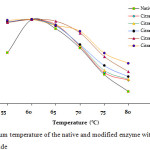 |
Figure 2: Optimum temperature of the native and modified enzyme with citraconic anhydride |
The result indicated that although there is no increase in the optimum temperature on the modified enzyme, but all modified enzymes have better acitivty at higher temperature than the native enzyme.
The effect of chemical modification to thermal stability
Figure 3 shows the residual activity of all enzyme at 60oC for 60 minutes.
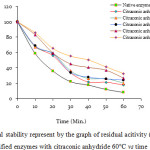 |
Figure 3: Thermal stability represent by the graph of residual acitivity (%) of the native and modified enzymes with citraconic anhydride 60°C vs time
|
The native enzyme after 60 minutes has residual activity of 8%, while the modified enzymes with citraconic anhydride with modification degree of 45, 73, 82, 84, and 88% have residual activity of 17.70; 20.24; 24.18; 26.70 and 32.6%, respectively.
There is increase on the thermal stability to the modified enzymes compared to the native enzyme. The thermal stability increase can be seen by the increase of residual activity of each enzyme which has incubated for 60 minutes. The chemical modification to native α-amylase used showed a good effect to the structure stability.
The mechanism of structure stability occured to α-amylase due to the chemical modification using citraconic anhydride can be explained that citraconic anhydride is a dicarboxylic acid, the carboxylic group will bind to ammine of the lysine residue on the surface of α-amylase15, so it will minimize contact of hydrophilic group with water as solvent, beside the enzyme structure became bigger so that the
Rate of thermal inactivation (ki), half-life (t1/2) and denaturation energy change (ΔGi) of native and modified enzymes
The values of rate of thermal inactivation (ki), half-life (t½) and denaturation energy change (ΔGi) of the native and modified enzymes with citraconic anhydride are shown in Table 1. The determination of ki value using the graph in Figure 4.
Table 1: The change of rate of thermal inactivation (ki), half-life (t½) and denaturation energy change (ΔGi) of the native and modified enzymes
| Enzyme | ki (1.min-1) | t1/2 (minute) | (ΔGi) (kJ/mol) |
| Native | 0.041 | 17 | 102.08 |
| Citraconic anhydride 45% | 0.030 | 23 | 102.91 |
| Citraconic anhydride 73% | 0.026 | 27 | 103.31 |
| Citraconic anhydride 82% | 0.024 | 29 | 103.53 |
| Citraconic anhydride 83% | 0,021 | 33 | 103.90 |
| Citraconic anhydride 88% | 0,018 | 39 | 104.33 |
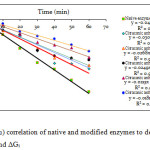 |
Figure 4: The Ln(Ei/E0) correlation of native and modified enzymes to determine the values of k1, t1/2 and ΔGi |
Table 1 shows the decrease of ki (thermal inactivation rate constant) values of all modified enzymes. The data indicated the decrease of denaturation rate or the increase of enzyme stability compared to the native enzyme.
The enzyme stability increase in this research based on the k1 values was 2-3 times compared to that of the native. The decrease of these k1 values was predicted due to the bond between ammine group on the side chain of lysine residue and carboxylate group on citraconic anhydride, as a result the native enzyme will be less flexible in water and the protein unfolding will also be decreased and caused the enzyme structure will be more rigid and become more stable16.
The modified enzymes using citraconic anhydride with modification degree of 45, 73, 82, 83, 88 % have raised the half-life from 17 minutes of the native enzyme to 23, 27, 29, 33 and 39 minutes, respectively. According Sthal17 the enzymes stability may be determined by their half-life.
The ΔGi value shows the energy used to denaturate the enzyme, the bigger the ΔGi value, the more stable the enzyme indicated that the enzyme is getting more difficult to denaturate. In this research, the increase of ΔGi values of modified enzyme were 0.5 – 1.2 times compared to the native enzyme as can be seen in Table 1. The higher of ΔGi values of the modified enzymes indicated that their stability was increasing.
Conclusions
Based on the results discussed above it was noted that the chemical modification with citraconic anhydride was able to increase the thermal stability of the native α-amylase from local bacteria isolate B. subtilis ITBCCB148 up to 2-3 times. The chemical modification employed has increased the thermal stability of the enzyme against pH and temperature, so there is possibility that the enzyme can be used in industrial process which require extreme condition.
Acknowledgments
The authors would like to thank to The Directorate of Research and Community Services, Directorate General of Higher Education, The Ministry of National Education of Republic of Indonesia that provides fund for this project to be undertaken through competency based research scheme (Hibah Kompetensi) with contract number of 133/SP2H/PL/Dit. Litabmas/III/2012, 7 Maret 2012
References
- Pandey, A., Nigam P. And Soccol C.R. Biotechnol Appl. Biochem., 31: 135-152 (2000).
- Maarel , M., Van der Veen, B., Uitdeehag, H., Lee,huis, H. And Dijkhuizen , L. J. Biotechnol., 94: 137-155 (2002).
- Wagen, E.S. Strategies for increasing the stability of enzymes, in Enzyme Engineering 7, The New York Academy of Sciences, New York, 1-19 (1984).
- Mozhaev, V.V. and K. Martinek. Enzyme Microb. Technol., 6 (2): 50-59 (1984).
- Dixon, H.B.F. and Perham, R.N. Biochem. J., 109: 312-314 (1968).
- Khajeh, K., Azadeh, E.H. and Mohsen, N.G. J. Biochem. Molecul. Biol., 37: 642-647 (2004).
- Yandri, Apriyanti, Suhartati, T. and Hadi, S. Biosci. Biotechnol. Res. Asia, 7 (2), 713 – 718 (2010).
- Yandri, Anggraini, N., Suhartati, T. and Hadi, S. Orient. J. Chem., 27 (3), 985 – 990 (2011).
- Yandri, A.S., Suhartati, T. and Hadi, S. Eur. J. Sci. Res., 39: 64-74 (2010). .
- Fuwa H., J. Biochem. (Tokyo), 41: 583-603 (1954).
- Mandels M., Raymond A. and Charles R., Biotech & Bioeng. Symp. No 6. John Wiley & Sons Inc (1976).
- Lowry O.H., Rosebrough N.J., Farr A.L. and Randall R.J., J. Biol. Chem., 193-269 (1951).
- Khajeh, K., Naderi-Manesh, H., Ranjbar, B., Moosavi-Movahedi, A. A. and Nemat-Gorgani, M. Enzyme Microb. Technol., 28: 543-549 (2001).
- Kazan D., Ertan H. and Erarslan A., Appl. Microbiol. Biotechnol., 48: 191-197 (1997).
- Janecek S., Proc. Biochem., 28 : 435-445 (1993).
- Yang Z., Michael D., Robert A., Fang X.Y. and Alan J.R., Enzyme Microb. Technol., 18: 82-89 (1996).
- Stahl S., Thermophilic Microorganisms: The Biological Background for Thermophily and Thermoresistance of Enzymes in Thermostability of Enzymes (Gupta, M.N. editor), Springer Verlag, New Delhi, 59-60 (1999).

This work is licensed under a Creative Commons Attribution 4.0 International License.









