Synthesis, Characterization and Antimicrobial Evaluation of some Schiff Bases and their Thiazolidinone Products
Desta Gebretekle, Abi Tadesse, R. K. Upadhyay* and Aman Dekebo
Department of Chemistry, College of Natural and Computational Sciences, Haramaya University, Ethiopia.
Article Received on :
Article Accepted on :
Article Published : 21 Oct 2016
Six isomeric nitro- and methoxy anilines were condensed with vanillin to obtain Schiff’s bases. A new series of 2-(4-hydroxy-3-methoxy phenyl)-1-thiazolidinone derivatives were synthesized by the cyclocondensation of Schiff’s bases with mercapto acetic acid. The chemical structures of synthesized compounds were confirmed by elemental analysis, molecular weight determination, IR, 1H & 13C and DEPT-135 NMR spectral measurements. Antibacterial and antifungal activities were studied in vitro against staphylococcus aurous and Escherichia coli bacteria and Aspergillus niger and Rhizoctoia bataticola fungi by using Ampicillin and Bavistin reference drugs respectively.
KEYWORDS:Schiff’s bases; Amines; Thiazolidinones; Bacteria; Fungi
Download this article as:| Copy the following to cite this article: Gebretekle D, Tadesse A, Upadhyay R. K, Dekebo A. Synthesis, Characterization and Antimicrobial Evaluation of some Schiff Bases and their Thiazolidinone Products. Orient J Chem 2012;28(4). |
| Copy the following to cite this URL: Gebretekle D, Tadesse A, Upadhyay R. K, Dekebo A. Synthesis, Characterization and Antimicrobial Evaluation of some Schiff Bases and their Thiazolidinone Products. Available from: http://www.orientjchem.org/?p=22762 |
Introduction
Schiff’s bases, characterized by the presence of imine or azomethine (-C=N-) group, constitute an important class of organic synthetic compounds. Imines owing to –C=N- linkage are useful as precursors in diverse organic synthesis and exhibit wide spectrum of biological properties, viz. pesticidal1, fungicidal2-4, bactericidal5,6, bacteriostatic7, anticancer8,9, antiviral10,11 etc. They have been reported as novel ligands informing complexes of unusual coordination numbers and isomeric structures with metals12, as dyes13 and analytical reagents 14, 15. Vanillin containing Schiff’s bases act as a weak inhibitor of tyrosinase and display both antimutagenic and comutagenic properties in Escherichia coli16 in addition to antimicrobial activities against various microbes and several pharmacological properties1.
Thiazolidinone derivatives were reported to display antitumor17, antituberculor18, anti-HIV19, analgesic20, anti-inflammatory20, ulcerogenic21, and antibacterial22 and antifungal22 activities. Therefore it was envisaged that compounds containing vanillin and thiazolidinone moieties would result in compounds of interesting biological properties. In the present study vanillin was treated with isomeric nitro-and methoxy anilines to produce Schiff’s bases. The Schiff’s bases were subjected to cyclocondensation reactions with thioglycolic acid to produce 4-thiazolidinones. The chemical structures of the synthesized compounds were confirmed by means of elemental analyses, molecular weight determination, and IR, 1H, & 13C and DEPT-135 NMR. All the compounds were screened against Gram-positive and Gram-negative bacteria and fungi.
Experimental
Synthesis Scheme
Synthesis of 1, 4-thiazolidinones involved two steps
Reagent And conditions
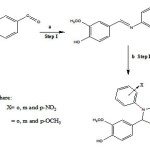 |
FIGURE 1:(a)Respective primary amines, dry ethanol, reflux 3h;(b) Thioglycolic acid, dry acetone, anhydrous CH3COONa, aqueous Na2CO3, reflux 3h Click here to View figure |
Step I
All the Schiff’s bases were prepared by mixing equimolar (0.5 mol) saturated solutions of each of substituted anilines with vanillin in dry ethanol and reaction mixtures were refluxed for 3 h on water bath. The solids precipitated or obtained on evaporation of the solvent as residues were washed with water and ether to remove unreacted vanillin and amine, if any, successively. Products were crystallized from acetone or methanol and dried in oven at 60-70 0C.
Step II
For the synthesis of 1, 4-thiazolidinones, saturated solution of Schiff’s bases (0.25 mol) in acetone was mixed with thioglycolic acid in 1:3 molar ratio and 5 gram anhydrous sodium acetate was added to the reaction mixture and refluxed for h. Hot reaction mixtures were filtered, cooled to room temperature and neutralized with aqueous Na2CO3 solution to remove unreacted acid; precipitates of products were filtered and washed with water repeatedly to ensure complete removal of sodium salt(s) and dried in air. All the compounds were purified by crystallization from ethanol or ether.
The purity of the synthesized compounds was checked by thin-layer chromatography and the impure samples were purified either by column chromatography or by washing with the solvent as identified by TLC.
Physico-Chemical And Microbial Analysis
Microanalaytical analyses were performed on a Vario-el III, elemental-R analyzer. Melting points were determined in open glass capillaries using MP-D Mitaruma Rikero Kiygo electro-thermal melting point apparatus. Molecular weights of the compounds were determined by Rast`s method with camphor as solvent. IR spectra in 500 cm-1 – 4000 cm-1 range were recorded in KBr medium on FT-IR Shimadzu spectrometer. 1H spectra, 13C and DEPT-135 NMR spectra were recorded in CDCl3 on Bruker 400 MHz Advanced spectrometer.
Antimicrobial (antibacterial and antifungal) activities of the azomethines and thiazolidinones, were tested in vitro using disc diffusion method against Staphylococcus aureus and Escherichia coli bacteria in Mueller Hinton Agar (MHA) medium and against Aspergillus niger and Rhizoctoia bataticola fungi in Potato Dextrose Agar (PDA) medium. The degree of bactericidal and fungicidal activities was determined by measuring diameter of inhibition zone and compared with the standard drugs ampicillin and bavistin, respectively.
Results And Discussion
Theoretically proposed molecular formulae of the compounds are in conformity of experimental data of molecular weights and elemental analyses (Table-1).
Table 1: Structure, melting point, yield and analyses data of compounds
| Compound | Colour | Yield(%) | M.P.(oc) | Molecular weight: Calcd.(Found) | Analyses: Calcd.(Found) % | |||
| C | H | N | S | |||||
|
1a |
Smoky white |
51.9 |
78±1 |
272 (264.7) |
61.76 (62.20) |
4.44 (4.90) |
10.29 (10.20) |
– |
|
1b |
Light yellow |
96.2 |
132±1 |
272 (273.8) |
61.76 (61.21) |
4.44 (4.62) |
10.29 (10.22) |
– |
|
1c |
White |
94.2 |
103±1 |
272 (283.6) |
61.76 (61.65) |
4.44 (4.68) |
10.29 (10.17) |
– |
|
1d |
Yellow |
94.0 |
119±1 |
257 (248.1) |
70.02 (69.76) |
5.88 (6.12) |
5.44 (5.33) |
– |
|
1e |
Light yellow |
58.4 |
126±1 |
257 (256.1) |
70.02 (69.26) |
5.88 (6.54) |
5.44 (5.82) |
– |
|
1f |
White |
99.2 |
154±1 |
257 (259.5) |
70.02 (69.80) |
5.88 (5.46) |
5.44 (5.40) |
– |
|
2a |
Yellow |
51.3 |
116±1 |
346 (345.2) |
55.48 (55.00) |
4.07 (4.24) |
8.09 (7.73) |
9.26 (9.05) |
|
2b |
Orange |
59.4 |
113±1 |
346 (360.9) |
55.48 (54.92) |
4.07 (4.18) |
8.09 (8.16) |
9.26 (9.36) |
|
2c |
Light yellow |
65 |
166±1 |
346 (336.4) |
55.48 (55.26) |
4.07 (4.41) |
8.09 (7.84) |
9.26 (9.10) |
|
2d |
Orange |
62.3 |
119±1 |
331 (317.6) |
61.61 (60.98) |
5.17 (5.31) |
4.23 (4.28) |
9.68 (9.35) |
|
2e |
Yellow orange |
62.1 |
134±1 |
331 (330.8) |
61.61 (59.87) |
5.17 (5.13) |
4.23 (3.98) |
9.68 (9.90) |
|
2f |
Pale orange |
81.5 |
141±1 |
331 (354.5) |
61.61 (60.69) |
5.17 (4.86) |
4.23 (3.99) |
9.68 (9.29) |
Where
C7H7O2-CH=N-ArX
C7H7O2-C3H3NOS-ArX
o-NO2
m-NO2
o-OCH3
m-OCH3
p-OCH3
The common characteristic groups of 1a-f and 2a-f of azomethines and thiazolidinones, display ν C-OH & δ C-OH (phenolic), ν C-O-C, aromatic ν C=C & ν C-H, aliphatic ν C-H, ν C-NO2 (symm.) & ν C-NO2 (asymm.) and ortho, meta and para substitution vibrations in their infrared spectra in 3215-3482 cm-1 & 1029-1154 cm-1, 1265-1378 cm-1, 1452-1595 cm-1 & 2954-3106 cm-1, 2921-3105 cm-1, 1265-1377 cm-1 & 1515-1666 cm-1, and ca.747-727 cm-1, ca.777-789 cm-1 and ca.843-836 cm-1 regions respectively. A strong band observed in 1a-f at 1592-1668 cm-1 range assigned to CH=N clearly indicates formation of the new azomethines owing to condensation of CHO group of vanillin with NH2 group of primary amine and absence of any peak of CHO group supports the proposed structure of 1a-f. Cyclocondensation of azomethine compounds with thioglycolic acid leads to the formation of thiazolidinones containing C=O, C-S-C, C-N and CH2 groups of heterocyclic ring with elimination of water. IR spectra of these compounds exhibit ν C=O (cyclic), ν C-S-C( ring) and ν C-N( ring) vibrations in 1608-1667 cm-1, 650-722 cm-1 and 1300-1367 cm-1 regions respectively whereas heterocyclic ring methylene group (CH2) displayed its ν C-H symmetric, ν C-H asymmetric and δ C-H bands in 2852-2855 cm-1, 2921-2955 cm-1 and 1447-1463 cm-1 ranges respectively. The absence of ν CH=N peak and presence of these characteristic groups of heterocyclic ring obviously conform the cyclization of the azomethines leading to the formation of thiazolidinones (2a-f)23.
For the verification of IR results, 1H and 13C NMR spectra of the compounds have been examined. 1H NMR spectra of azomethines (1a-f) display singlet in δ 3.80- 4.00, δ 7.70-8.40 and δ 8.40- 9.85 regions characteristics of –OCH3, -OH and –CH=N groups, respectively and multiplet bands of benzene rings in δ 6.27- 8.06 range. 1H NMR spectra of thiazolidinones however displayed signals in δ 3.85- 4.40, δ 8.10- 9.85, δ 8.10- 8.75 and δ 5.55- 6.20 regions for their OCH3, OH(phenolic), CH-N (ring) and -S-CH2 (ring) groups respectively; benzene protons exhibit multiplets in δ 6.10-7.60 ppm region24. 13C NMR spectra of 1a-f of carbon containing groups-OCH3, C-OH, CH=N and C-NO2 exhibit their characteristic signals in δ 56, δ 147-153, δ 159-191 and δ 136-147, respectively along with benzene ring carbons in δ 107-161 range.13C NMR spectra of 2a-f display signals of carbon containing groups (Ar)O-CH3, C-OH (phenolic), C-N (ring), C=O (ring), C-S (ring) and CH2 (ring) in δ 56-66, δ 139-152, δ 55-56, δ 166-191, δ 55-56 and δ 30-34 ranges respectively and aromatic carbon signals in δ 109-158 region. In NO2 substituted products C-NO2 signal is exhibited in δ 136-148 range. Signals corresponding to C-N (ring), C-S (ring) and O-CH3 group have been seemed to be overlapped. All the NMR inferences are totally in conformity of IR results25.DEPT-135 spectra of azomethines also displaying O-CH3 and CH=N signals at the same ppm as observed in 13C NMR spectra and seven aromatic hydrogen bonded carbons exhibit seven peaks in upward direction as expected. Thiazolidinones also display peaks of OCH3, CH-N and aromatic hydrogen bonded carbons exhibit peaks at the same ppm as observed in13C NMR spectra in upward direction as expected25.
The results of antimicrobial studies reveal less significant antibacterial activity of both series of compounds, against both test bacteria; m- methoxy thiazolidinone however exhibits better results against E.coli among all others. All the compounds show better antifungal activities than bactericidal properties against both fungi tested. Although several azomethines and thazolidinones exhibit highly significant results, p-nitro thiazolidinone has highest antifungal action against R. bataticola in both the concentrations used, better than standard drug bavistin (Table 2)26.
Table 2: Inhibitory zone diameters (%) of azomethines and thiazolidinones against tested bacteria and fungi strains by paper disc diffusion method
|
Compound |
Antibacterial activity |
Antifungal activity |
||||||
| S. aureus | E.coli | A.niger | R.bataticola | |||||
| 10 μL/ disc | 20 μL/ disc | 10 μL/ disc | 20 μL/ disc | 10 μL/ disc | 20 μL/ disc | 10 μL/ disc | 20 μL/ disc | |
| 1a | 32 | 32 | 27 | 27 | 30 | 36 | 39 | 39 |
| 1b | 32 | 36 | 27 | 31 | 65 | 59 | 58 | 65 |
| 1c | 32 | 36 | 23 | 27 | 50 | 73 | 58 | 65 |
| 1d | 32 | 32 | 23 | 27 | 30 | 36 | 65 | 62 |
| 1e | 32 | 36 | 23 | 31 | 40 | 41 | 54 | 65 |
| 1f | 36 | 36 | 23 | 27 | 30 | 32 | 58 | 73 |
| 2a | 32 | 41 | 27 | 31 | 40 | 50 | 50 | 62 |
| 2b | 36 | 45 | 31 | 35 | 45 | 55 | 54 | 50 |
| 2c | 32 | 41 | 27 | 35 | 70 | 64 | 100 | 115 |
| 2d | 32 | 41 | 27 | 31 | 30 | 36 | 23 | 35 |
| 2e | 36 | 45 | 39 | 50 | 45 | 41 | 27 | 35 |
| 2f | 36 | 41 | 31 | 42 | 30 | 27 | 31 | 35 |
|
DMSO |
– | – | – | – | – | – | – | – |
|
Ampicillin |
100 | 100 | 100 | 100 | – | – | – | – |
| Bavistin | – | – | – | – | 100 | 100 | 100 |
100 |
References
- Thorat B.R, Mandewala M, Shelke S, Kamat P, Atram R.G, Bhalerao M, Yamgar R. J. Chem. Pharm.Res. 2012;4:14.
- Singh H, Yadav L.D.S, Mishra S.B.S. J. Inorg. Nucl. Chem. 1981;43:1701.
- Saravanan G, Pannerselvam P, Prakash C.R. J. Adv. Pharm. Techn. Res. 2010;1: 320.
- Panneerselvam P, Nair R.R, Vijayalakshmi G, Subramanian E.H, Sridhar S.K. Eur. J.Med. Chem., 2005;40:225.
- Przybylski P, Huczynski A, Pyta K, Brzezinski B, Bartl F. Curr. Org. Chem., 2009;13:124.
- Karthikeyan M.S, Parsad D.J, Poojary B, Bhat K.S, Holla B.S, Kumari N.S. Bioorg. Med. Chem., 2006;14:7482.
- Yuxia Z, Tao Z, Wanshan M, Haibin Z, Suifeng C. Hauxue Shiji, 2002;24:117
- Sinha D, Tiwari A.K, Singh S, Shukla G, Mishra P, Chandra H, Mishra, A.K. Eur. J.Med. Chem. 2008;43:160
- Przybylski P, Pyta K, Wicher B, Gdaniec M, Brzezinsk B. J. Mol. Struct. 2008;889:332.
- Holla B.S, Akberali P.M, Shivananda M.K. Il Farmaco. 200156:919.
- Jarrahpour A, Khalili D, De Clercq E, Salmi C, Brunel J.M. Molecules, 2007;12:1720.
- Upadhyay R.K, Sharma V.K., Singh V.P. J. Liq. Chromatography. 19825:1141.
- Upadhyay R.K, Agarwal. N. Nat. Acad. Sci. Letter, 1991;14: 251.
- Upadhyay R.K, Bajpai A.K, Rathore K. Chromatographia. 1984;18:618.
- Wtanable K, OhtaT, Shirasu,Y. Mutat Res. 1989;218:105.
- Mehta D.S, Shah V.H. Indian. J.Heterocyc.Chem. 2001;11:139-144.
- Kucukguzel S.G, Orul E.E, Rollas S, Salin F, Ozbek A. Eur.J.Med.Chem. 2002;37: 197-206.
- Rawal R.K, Prabhakar Y.S, Katti S.B, DeClercq E. Bioorg.Med.Chem. 2005;13:6771-6776.
- Vigorita M.G, Ottana R, Monforte F, Maccari R, Trovato A, Monforte, M.T, Taviano, M.F. Bioorg.Med. Chem.Lett. 2001;11:2791-2794.
- Shanmugapandiyan P, Denshing K.S, Ilavarasan R, Anbalagan N, Nirmal R. International Journal of Pharmaceutical Sciences and Drug Research. 2010;2:115-119.
- Singh G.S, Molotsi B.J. Il Farmaco. 2005;60:727-730.
- Rahman V. P. M, Mukhtar S, H.Ansari W, Lemiere G. Eur.J.Med.Chem. 2005;40: 173-184.
- Timothy D.W. High-Resolution NMR Techniques in Organic Chemistry 1st edition (1999).
- Meyers R.A, Wiley and Sons, Ó J. Interpretation of Infrared Spectra, Ltd a Practical Approach, 2000;10815–10837.
- Hore P.J, Oxford University Press, USA; Nuclear Magnetic Resonance. 1st edition (1995).
- Yadav P.S, prakash D, Senthilkumar, G.P. Intern. J. Pharm. Sc. Drug Res. 2011;3:01-07.

This work is licensed under a Creative Commons Attribution 4.0 International License.









