Micellar Mediated Dissolution of a Hydantion Drug
Pinki Sharma and L.K. Tiwary*
Regional Institute of Education, NCERT, Bhopal - 462 001, India.
Article Received on :
Article Accepted on :
Article Published : 31 Dec 2012
Dissolution behaviour of both 5, 5-diphenyl hydantoin sodium commonly known as dilantin sodium (DS) and its corresponding marketed capsule dilantin have been studied in aqueous as well as micellar media. Percentage dissolution of both DS and its marketed capsule are found to be low as expected in aqueous medium due to its very low solubility in water. However, their dissolution tendency is found to be significantly enhanced in presence of sodium dodecyl sulphate (SDS) and cetyltrimethylammonium bromide (CTAB). Impact of SDS and CTAB on the dissolution behaviour of pure DS and on the dilantin capsule are observed to be nearly same.
KEYWORDS:Dilantin sodium Percentage dissolution; Sodium dodecyl sulphate; Cetyltrimethylammonium bromide
Download this article as:| Copy the following to cite this article: Sharma P, Tiwary L. K. Micellar Mediated Dissolution of a Hydantion Drug. Orient J Chem 2012;28(4). |
| Copy the following to cite this URL: Sharma P, Tiwary L. K. Micellar Mediated Dissolution of a Hydantion Drug. Available from: http://www.orientjchem.org/?p=22865 |
Introduction
Drug dissolution can be defined by the extent and the rate of dissolution and involves two steps, drug release from the dosage form (liberation process) and drug transpot within the dissolution medium (convection proces). The rational behind the dissolution test is that a drug should be appropriately dissolved within the gastrointestinal tract (GIT) in order to be absorbed. Dissolution hence has become the most important test to know the product quality and drug release behaviour in addition to other applications.1-5
Several factors influence drug dissolution including physicohemical properties of drug, formulation characteristics, dissolution method etc.6 Furthermore, the G1 condition must be maximally simulated in a well-designed dissolution testing. An appropriate dissolution method also should be discriminative which means it should be sensitive to product quality in terms of release characteristics and provide reproducible results.
Dissolution study is particularly important for insoluble or low solubility drugs, where absorption is dissolution rate limited. At the same time, development of a dissolution method for this group of drugs in very challenging. Dissolution medium must provide sink conditions. Absence of sink conditions may result in unpredictable release kinetics and suppression of release profiles. Different techniques like use of large dissolution volume, removal of dissolved drug, pH changes, and addition of surfactants or their combination have been employed by scientist to improve solubility and ensure sink conditions7. Out of all the above approaches pH modification and surfactant addition appear to be the simplest and can be tailored to resemble G1 fluid environment.
An important property of surfactants is their ability to form colloidal – sized aggregates in aqueous solution, known as micelles. Micelles are particularly useful in pharmaceutical applications because of their ability to increase the solubility of sparingly water-soluble substances. Correlating the above context, solubilization is defined as the spontaneous dissolution of a material by reversible interaction with micelle to form thermodynamically stable isotropic solution that exhibits reduced thermodynamic activity of the solubilized material9. The spatial position of a drug solubilized with a micelle depends on the drug polarity.
Numerous drug delivery and drug targeting system have been studied in an attempt to minimize drug degradation and loss, to prevent harmful side effects, and to increase drug bioavailability10-14. The utilization of micelles as drug carriers in aqueous media presents some advantages when compared to other alternatives such as the use of water soluble polymers and liposomes. Micellar systems can solubilize drugs that are poorly soluble in water, increasing bioavailability by lengthening retention in the body to provide gradual accumularation in the required area. In addition, the small size of micelles permits them to accumulate in area with leaky vasculature15.
Hydantion drugs are known for their antimicrobial anticonvulsant antimicrobial anticonvulsant properties and are commonly used antiepileptical drugs16. Surface activity of some of the hydantoin drugs have recently been investigated17-18. Dilantin sodium (I) is an important hydantoin class of drug and is a commonly used antiepileptic which acts to suppress the abnormal brain activity seen in seizures by including electrical conductance among cells by stebilizing the inactive state of voltage gated sodium channels. Recently we have investigated aggregation behaviour of dilantin sodium in aqueous as well as in micellar media and also its interaction with common surfactants like SDS and CTAB19.
This is for the first time as per available literature that we have made an attempt to investigate the dissolution behaviour of dilantin sodium and marketed medicine dilantin in aqueous as well as micellar media.
Materials And Methods
Dilantin sodium and also its marketed drug dilantin are seen to show max at 216 nm. Caliberation curve was prepared by determining absorbance of five different solutions of pure DS with 5, 10, 15, 20 and 25 ug/mL concentrations. Slope of the calibration curve was determined utilizing regression equation. Volume of 900 mL was maintained in dissolution apparatus for making dissolution study. A fixed amount of drug solution was withdrawn from dissolution apparatus at regular interval and after making proper dilution their absorbances were noted. Percentages dissolution (uncorrected) was calculated utilizing the formula
|
% dissolution |
= |
Absorbance |
x |
dilution factor |
x 900 x |
100 |
|
(uncorrected) |
slope |
1000 |
amount of |
|||
|
|
|
|
|
|
|
drug used |
In this study the dilution factor maintained is 5 in all cases. Correction factor was calculated by utilizing the following relations.
|
Correction factor = |
Percentage dissolution (uncorrected) |
× |
Volume of sample withdrawn at regular interval |
|
900 |
This correction factor is added in the next uncorrected percentage dissolution to obtain corrected percentage dissolution. In order to maintain proper G1 environment all measurement are made in acidic condition and at 37oC.
Both dissolution apparatus and uv-visible spectrophotometer used are of LABINDIA MAKE. Rotation per minutes (RPM) of 50 is maintained in all such dissolution study. A 100 mg. capsule have been used to study its dissolution behaviour.
Results & Discussion
Pure dilantin sodium shows distinct max at 216 nm in aqueous environment and its marketed capsule dilantin is also seen to absorb at the same wavelength. Hence for preparing calibration curve pure compound has been utilized. A stock solution of 1000 ppm of pure DS was prepared in acidic dissolution media and this solution was diluted to obtain solution of 5, 10, 15, 20 and 25 g/mL concentration. Absorbance of these solutions were determined and plotted against the concentrating of solution to obtain calibration curve. The data obtained in shown in table 1 and corresponding calibration curve is presented in figure 1.
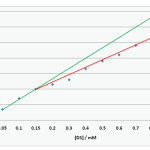 |
Figure 1:CMC of DS by conductivity method Click here to View figure |
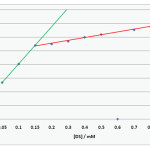 |
Figure 2:CMC of DS by Spectrophotometric method Click here to View figure |
In this study the dilution factor maintained is 5 in all cases. Correction factor was calculated by utilizing the following relations.
| Correction factor = | Percentage dissolution (uncorrected) | × | Volume of sample withdrawn at regular interval |
| 900 |
This correction factor is added in the next uncorrected percentage dissolution to obtain corrected percentage dissolution. In order to maintain proper G1 environment all measurement are made in acidic condition and at 37oC.
Both dissolution apparatus and uv-visible spectrophotometer used are of LABINDIA MAKE. Rotation per minutes (RPM) of 50 is maintained in all such dissolution study. A 100 mg. capsule have been used to study its dissolution behaviour.
Results & Discussion
Pure dilantin sodium shows distinct max at 216 nm in aqueous environment and its marketed capsule dilantin is also seen to absorb at the same wavelength. Hence for preparing calibration curve pure compound has been utilized. A stock solution of 1000 ppm of pure DS was prepared in acidic dissolution media and this solution was diluted to obtain solution of 5, 10, 15, 20 and 25 g/mL concentration. Absorbance of these solutions were determined and plotted against the concentrating of solution to obtain calibration curve. The data obtained in shown in table 1 and corresponding calibration curve is presented in figure 1.
Table :1 Calibration Curve of DS
|
[DS] / mg mL-1 |
Absorbance |
|
5 |
0.125 |
|
10 |
0.216 |
|
15 |
0.345 |
|
20 |
0.455 |
|
25 |
0.511 |
The slope of the line has been determined utilizing regression equation and is found to be 0.021.
The dissolution study of the drug has been carried out in two parts. In the first part dissolution behaviour of dilantin capsule is investigated and in the second part dissolution tendency of sprangily soluble DS has also been found out.
Dissolution behaviour of dilantin capsule.
The dissolution behaviour of dilantin capsule have been investigated in aqueous as well as micellar media. The concentration of surfactants have been maintained above their critical micelle concentration (CMC) in all dissolution studies. The corrected percentage dissolution measured in aqueous as well as in presence of SDS and CTAB are presented in Table. 2.
Table 2 : Corrected percentage dissolution of dilantin capsule (RPM = 50, volume = 900 mL).
|
Time |
Absorbance |
Corrected % dissolution |
||||
|
(minutes) |
Aqueous |
CTAB |
SDS |
Aquous |
CTAB |
SDS |
|
5 |
0.115 |
0.145 |
0.158 |
24.61 |
31.07 |
31.07 |
|
10 |
0.139 |
0.217 |
0.249 |
29.92 |
46.67 |
53.54 |
|
15 |
0.21 |
0.308 |
0.307 |
45.30 |
66.43 |
66.27 |
|
20 |
0.279 |
0.305 |
0.308 |
60.34 |
66.15 |
66.85 |
|
25 |
0.306 |
0.298 |
0.308 |
66.46 |
65.01 |
67.22 |
|
30 |
0.295 |
0.299 |
0.307 |
64.46 |
65.58 |
67.37 |
|
40 |
0.368 |
0.303 |
0.304 |
67.60 |
66.80 |
67.09 |
|
45 |
0.308 |
0.306 |
0.307 |
67.97 |
65.75 |
65.75 |
The data presented in Table 2 clearly indicates that surfactants CTAB and SDS are playing significant role in the dissolution of drug. In fifteen minutes only 44% of the drug is dissolved in aqueous medium whereas this value is more than 65% in presence of SDS and CTAB. Secondly, in all cases dissolution rate is limited after certain time interval, for example in aqueous medium increase in dissolution is negligible after 40 minutes whereas in presence of SDS and CTAB dissolution rate is limited only after 15 minutes. This may be explained on the basis of saturation solubility of the drug. Due to low solubility of drug its saturation solubility may also have lower value and use of 100 mg capsule may not completely dissolve in the experimental dissolution volume of 900 mL.
Dissolution behaviour of dilantin sodium
Dissolution behaviour of pure dilantin sodium have also been studied adopting the similarly procedure as discussed above. The compound has been taken in powdered form. The corrected percentage dissolution has been presented in Table 3.
Table-3 : Corrected percentage dissolution of dilantin sodium(RPM = 50, Volume = 900 mL)
|
Time |
Absorbance |
Corrected % dissolution |
||||
|
(minutes) |
Aqueous |
CTAB |
SDS |
Aquous |
CTAB |
SDS |
|
5 |
0.205 |
0.218 |
0.219 |
43.93 |
46.71 |
46.93 |
|
10 |
0.248 |
0.259 |
0.269 |
53.38 |
55.76 |
57.90 |
|
15 |
0.297 |
0.302 |
0.295 |
64.18 |
65.28 |
63.79 |
|
20 |
0.302 |
0.302 |
0.301 |
65.60 |
65.64 |
65.43 |
|
25 |
0.306 |
0.306 |
0.305 |
66.82 |
66.85 |
66.64 |
|
30 |
0.302 |
0.306 |
0.299 |
66.33 |
67.22 |
65.72 |
|
40 |
0.306 |
0.298 |
0.301 |
67.54 |
65.87 |
66.51 |
|
45 |
0.307 |
0.298 |
0.301 |
65.75 |
65.73 |
65.75 |
It is evident from the data presented above that dissolution of drug molecules is assisted in the micellar media but impact of SDS or CTAB is seen to be less as compared to their impact on the dilantin capsule. This clearly indicates that surfactants are making significant role on the drug release kinetics in their dosage form. Secondly, in the powder for also the dissolution of drug finds a limiting value above which increase in the dissolution is negligible. This behaviour is again explained on the basis of saturation solubility which may be low due to its very low solubility in aqueous medium.
Conclusion
Dissolution of dilantin in the oral dosage form (in the form of 100 mg. capsule) is seen to be significantly assisted by surfactants like SDS & CTAB. The effect of SDS and CTAB influencing dissolution of drug are nearly same. The dissolution of pure dilantin sodium is also enhanced by micellar media but the extent of assistance in lesser in this case compared to marketed dilantin capsule. However, in both dilantin capsule and pure dilantin sodium dissolution rate is limited after a certain interval of time.
Acknowledgement
Principal, RIE, Bhopal is thankfully acknowledged for providing laboratory facilities.
Reference :
- Schewartz, A.M., Parry, J.W., and Berck, J., Surface Active Agents and Detergents, 2 Interscience Publishers Inc., New York, 1949.
- Kuppusami, J. and Suryanarayana, C.V., Nature 208, 780 (1965).
- Chhatterjee, A., Moulik, S.P., Sanyal, S.K. Mishra, B.K. and Puri, P.M., J. Phys. Chem. B., 105, 12823 (2001)
- Moulik, S.P., Current Sci., 71, 368 (1996).
- Taboada, P., Gutierrez – Pichel, M., Barbosa, S., and Mosquera, V., Phy. Chem. Chem. Phys., 6, 5203 (2004)
- Taboada, P., Gutierrez – Pichel, M., Barbosa, S., and Mosqherra, V., Chem. Phys., 298, 65 (2004).
- Alam, M.S., Mandal, A., and Mandal, A.B., J. Chem. Eng. data, 56 (4), 1540 (2011).
- Alam, M.S., Samant, D. and Mandal, A.B., Colloid and Surfaces B: Bio-interfaces, 95, 203 (2012).
- Ghosh, K.K., Vidya, J. and Bal, S., J. Indian Chem. Soc., 82, 743 (2005).
- Alam, M.S., Ghosh, S., Mandal, A.B., and Kabir-ud-Din, Colloid and Surfaces B., 88, 779 (2011).
- Alam, M.S. and Mandal, A.B., J. Mol-Liquids, 168, 75 (2012).
- Kabir-ud-Din, Rub, M.A. and Nagri, A., J. Phys. Chem. B, 114, 6354 (2012).
- James, J., Vallaichemi, S., Krishnan, RSG, Samikannu, S., and Mandal, A.B., Chemical Physics, 312, 275 (2005).
- Michali, G., Linthicum, K., Han, N., Pharmacotherapy, 19, 223 (1999).
- Krishnan, R.S.G., Thennarasu, S., and Mandal, A.B., Chemical Physics, 291, 195 (2003).
- Tiwary, L.K., Mandal, A., Alam, M.S., Thennarasa S., and Mandal, A.B., Colloid and Surfaces B., Biointerfaces, 82, 126 (2011).

This work is licensed under a Creative Commons Attribution 4.0 International License.










